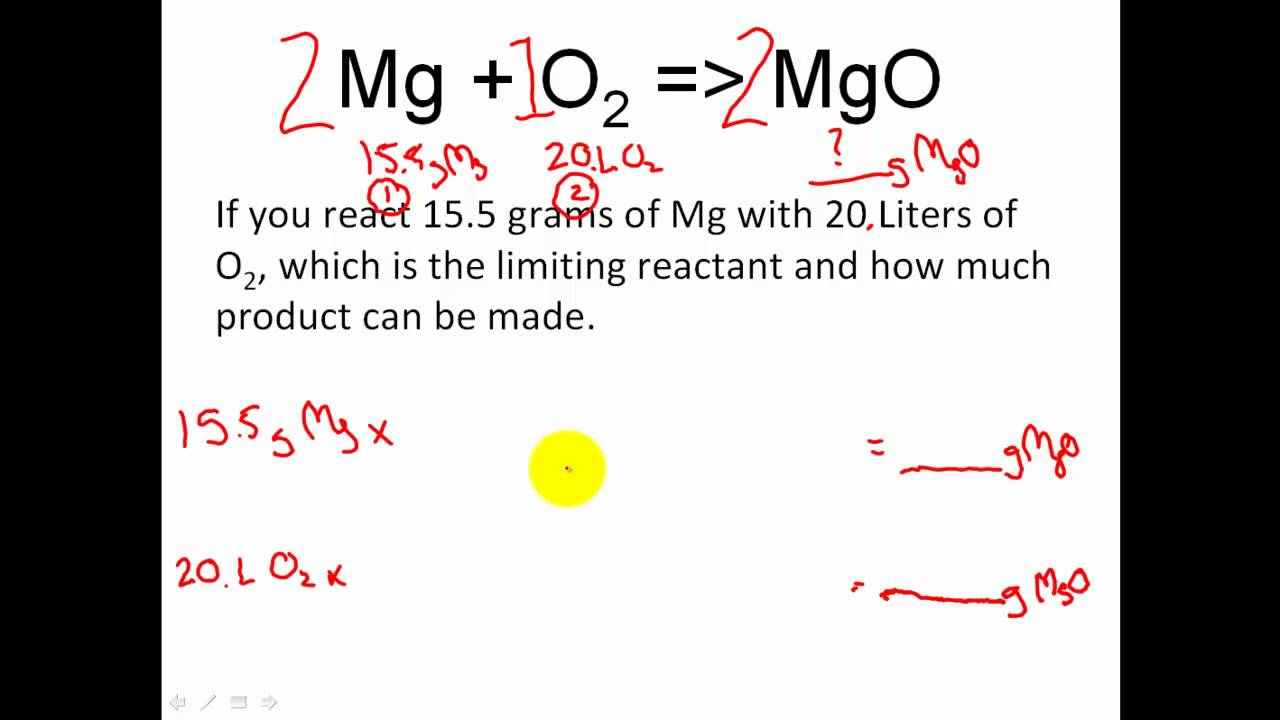Outrageous Reactant And Product Examples

Heresodium and chlorine are reactants and sodium chloride is product.
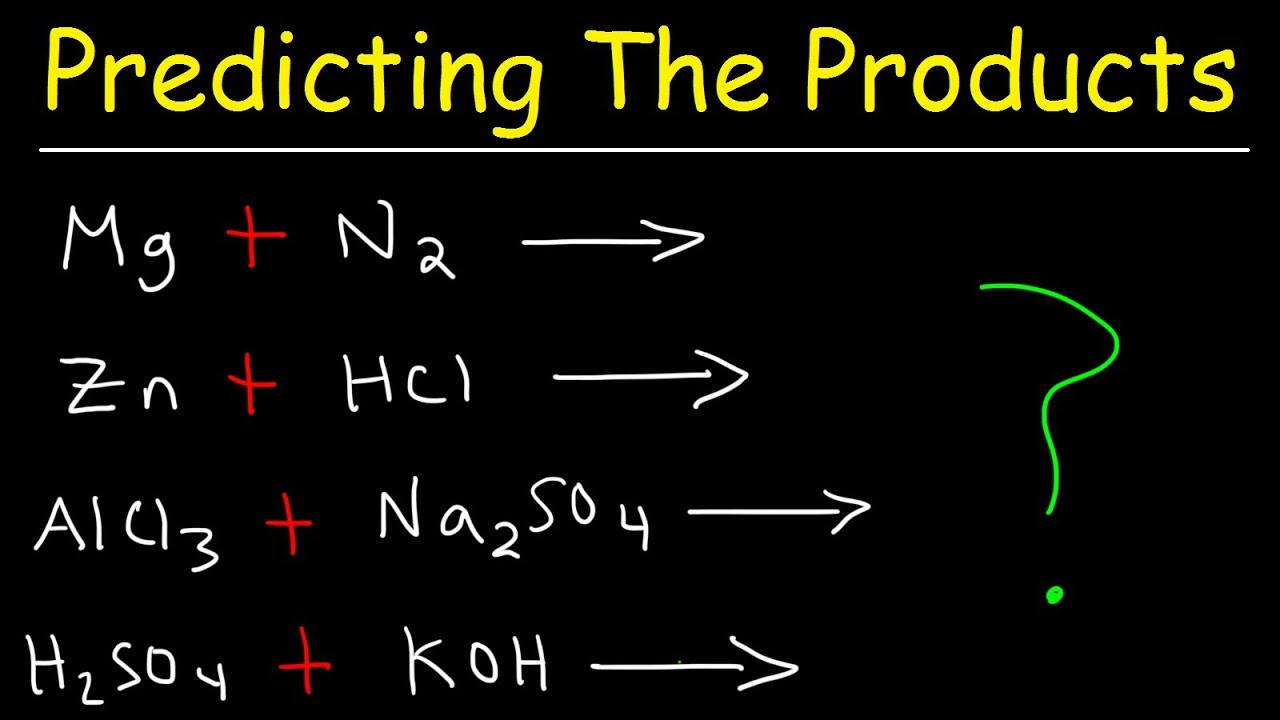
Reactant and product examples. Reactants are the starting material of a chemical reaction. Sodium sulfuric acid - sodium sulfate hydrogen Step 1 write formulas. They indicate the number of each chemical species that reacts or is formed.
A reactant is a chemical substance that is present at the beginning of the reaction and is featured on the left side of the reaction equation. Examples of Reactants A general reaction may be given by the equation. Explain the term reactant and product giving examples.
A reactant is a substance that is present at the start of a chemical reaction. On the contrary the products in. In the equation above the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product.
In the example of the candle burn the reactants are the fuel the candlewick and wax and oxygen present in air. There are various types of chemical reactions such as acid-base reactions redox reactions and combustion reactions. What are the products and reactants in this reactions.
A product is a substance that is present at the end of a chemical reaction. The substance which take part in a chemical reaction are called reactants. It will be clear by a simple reaction H2SO42NaOHNa2SO42H2O.
Productreactant x molar mass of productmole product Amount of Na 3 PO 4 formed from 3560 grams of NaOH. Another example of where we see a chemical reaction taking place is when we burn wood in a fire either in our homes or to cook food. Methane and oxygen oxygen is a diatomic two-atom element are the reactants while carbon dioxide and water are the products.