First Class Butane Incomplete Combustion

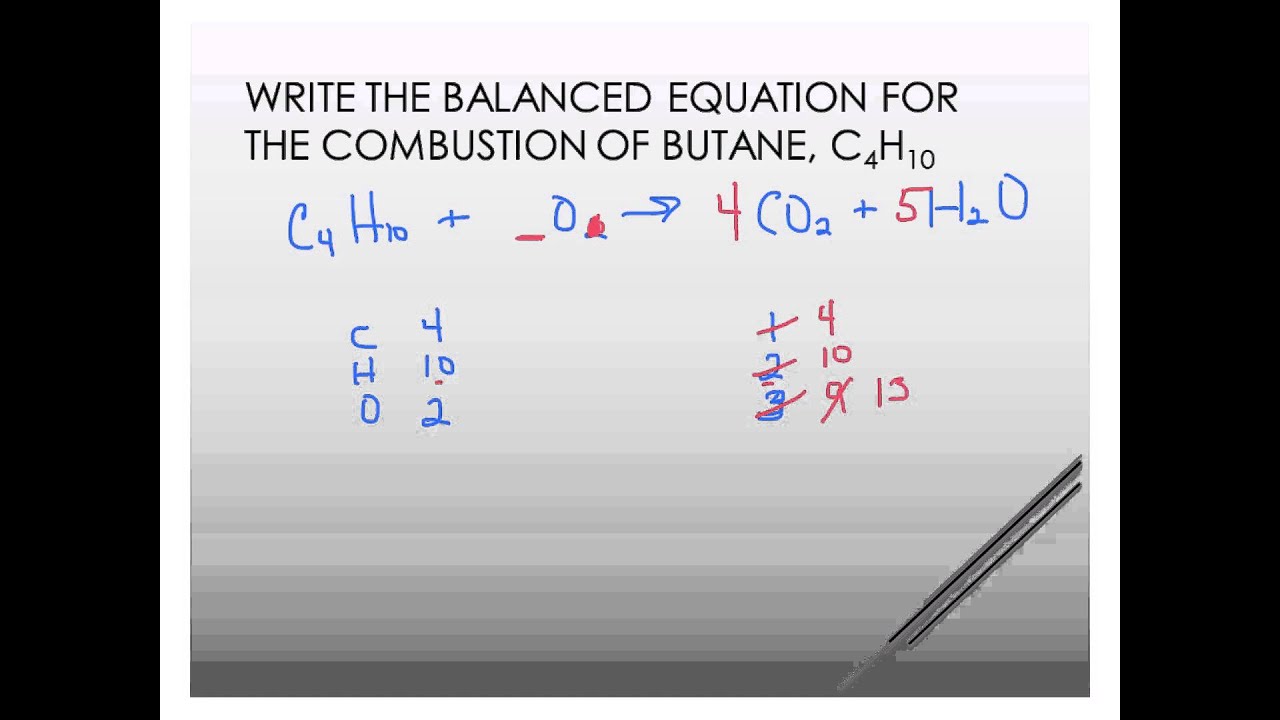
C4H10 132O2 4CO25H2O Therefore for combustion of 1 mole of butane 65132 moles of oxygen is required.
Butane incomplete combustion. I need to write an equation for the incomplete combustion of butane to form a mixture of carbon monoxide unburnt carbon and water. _____reading the eq _____2 C4H10 g________ 9 O2 g --------- 8 CO g. 65 moles of oxygen The combustion of butane is given by.
Soot hydrogen and nitrogen oxides are other common byproducts of incomplete combustion. There tends to be more incomplete combustion with long chain hydrocarbons than short chain ones. Either Butane itself is littered or the items it is used for -The reaction of butane combustion can also cause great damage to the environment.
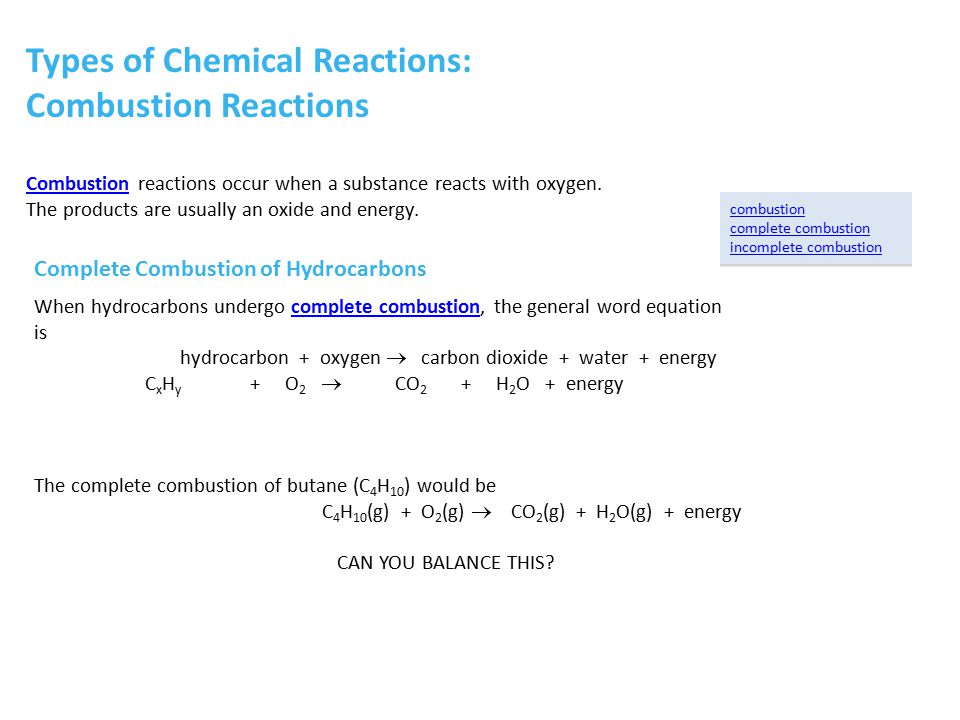
Butane is a straight chain alkane composed of 4 carbon atoms. When there is enough oxygen present in the surrounding butane can undergo complete combustion forming carbon dioxide and water vapor along with heat energy. Incomplete combustion is defined as the type of reaction in which a hydrocarbon reacts with oxygen to produce carbon monoxide water and carbon.
A complete combustion of butane b complete combustion of ethanol c incomplete combustion of cyclopentane Cracking Write a balanced molecular equation showing the formation of ethene from the cracking of hexane. Water is still produced but carbon monoxide and carbon are produced instead of carbon dioxide. Incomplete combustion of butane.
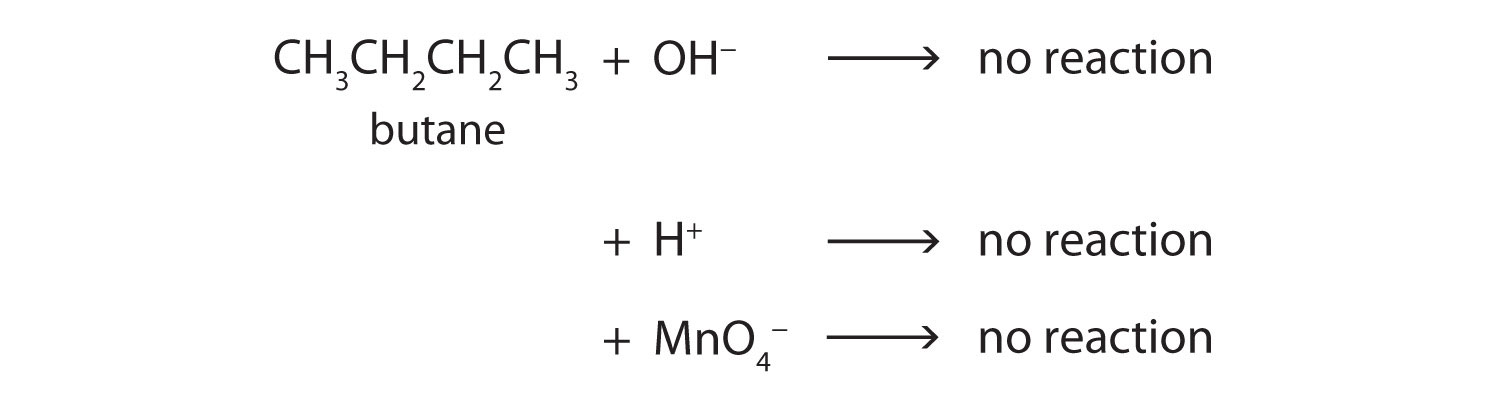
Transcribed image text. N 3 3 Substitution Reactions Complete the following reactions. An equation for the incomplete combustion of butane in oxygen is.
There tends to be more incomplete combustion with long chain hydrocarbons than short chain ones. Look for the differences. 1 Combustion Reactions Write balanced equations for the.