Favorite Iron Corrosion Equation

Corrosion is defined as the oxidation of metal by air.
Iron corrosion equation. The corrosion current in amperes. We can substitute in the value of Faradays constant. The thermodynamic considerations for different possible reactions for a metalH 2 O system can be summarized in potentialpH diagrams the so-called Pourbaix diagrams such diagrams were compiled by Marcel Pourbaix in 1963.
The formula is approximately Fe 2 O 3 3 2 H 2 O although the exact amount of water is variable. A Mathematical Model for the Corrosion of Iron in Sulfuric Acid E. Charge is given by Q I t where t is the time in seconds and I is a current.
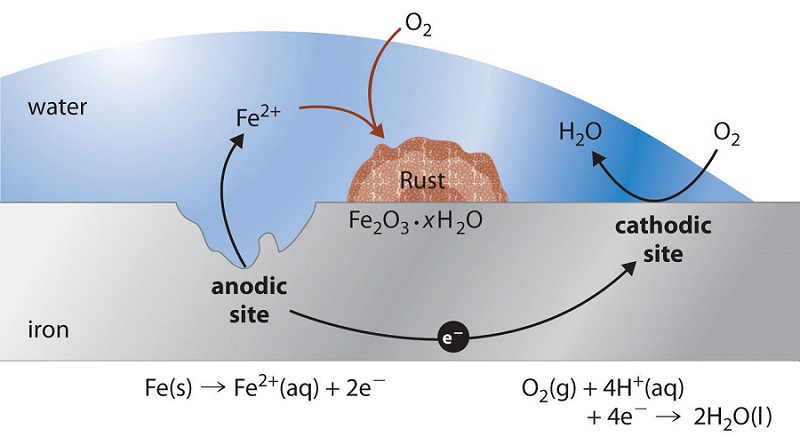
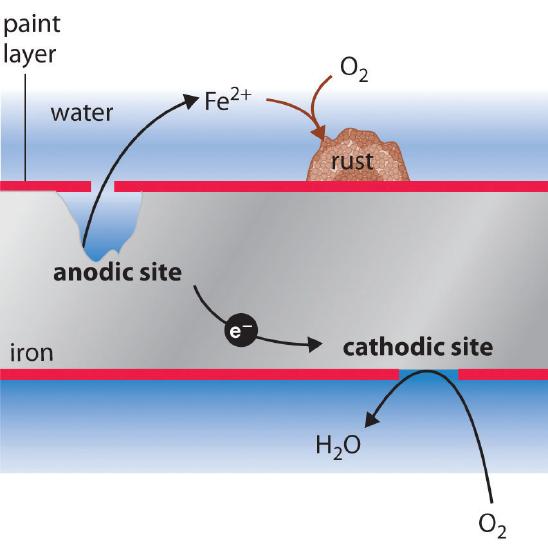
2Fe s O 2 g H 2 O l Fe OH 2 The iron II hydroxide is converted to rust through a serious of reactions. Figure 1 shows as an example the Pourbaix diagram for iron. The chemical formula of this compound is Fe 2 O 3.
I c K c exp α cE kT 15 For metallic iron in. What kinds of chemical treatments surface coatings or combinations of metals will. The overall chemical equation for the formation of rust is Iron water oxygen rust 4 Fe s 6 H 2 O l 3 O 2 g 4 Fe OH 3 s Iron III hydroxide Fe OH 3.
Gan and Mark E. Where m m0 - mt t is corrosion time measured at initial corrosion through metal resistance measuring A is surface area of the metal wire mo is initial metal wire masses and mt metal masses measured at time t. The iron II hydroxide firstly oxides to iron III oxide.
Rusting occurs when iron or steel reacts with oxygen and water. Httpsbitly3akrBoz to get all learning resources as per ICSE CBSE IB Cambridge. The chemical formula for rust is Fe2O3 and is commonly known as ferric oxide or iron oxide.



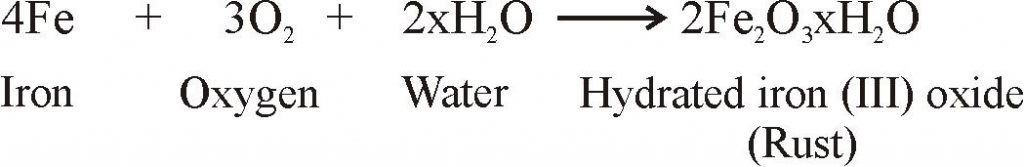




:max_bytes(150000):strip_icc()/BalanceEquations4-56a132763df78cf772685182.png)
