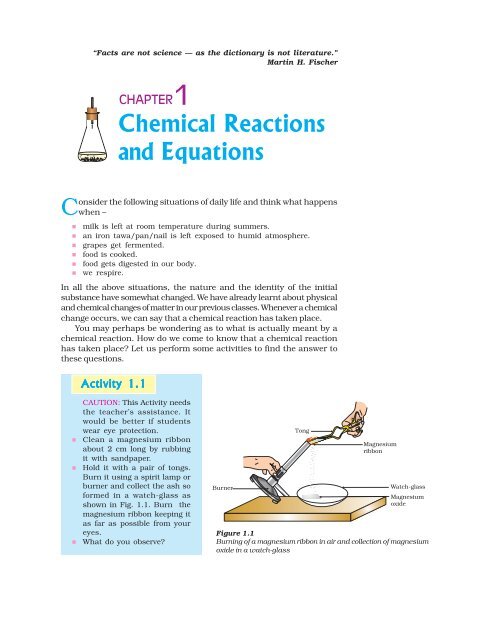
Exemplary Write A Chemical Equation For Endothermic Reaction And Make It More Informative

Updated May 09 2019.
Write a chemical equation for endothermic reaction and make it more informative. This is done as follows. Writing the condition in which reaction takes place. The arrow indicates the product and the direction of the reaction.
TO MAKE EQUATIONS MORE INFORMATIVE. The chemical equation can be made more informative. Using chemical formulas instead of words can make chemical formulas more concise and useful.
The reactions in which heat is absorbed are called endothermic reactions. This is done as follows. The total chemical energy of the products is higher than that of the reactants.
An endothermic process or reaction absorbs energy in the form of heat endergonic processes or reactions absorb energy not necessarily as heat. An endothermic Reaction by indicated by writing Heat or Heat energy Or just energy on the reactant side of the equation. Is provided above the arrow.
Chemical reactions proceed with the evolution or absorption of heat. The information regarding temperature pressure and catalyst etc. Physical state Information about the physical states of reactants and products can be notations sl g and aq for solids liquids gases and substance in water respectively along with their chemical formulae.
For example-Respiration burning of fuel Rusting of iron photosynthesis Digestion of food etcREACTANTS AND PRODUCTS. These examples could be written as chemical reactions but are more generally considered to be endothermic or heat-absorbing processes. The reaction equationNH4NO3s water NH4aq NO3aq This is an example of endothermic reaction because the temperature drops because heat energy is taken in by the reaction mixture.