Top Notch Balanced Decomposition Reaction

You balance decomposition reactions just like any other reaction.
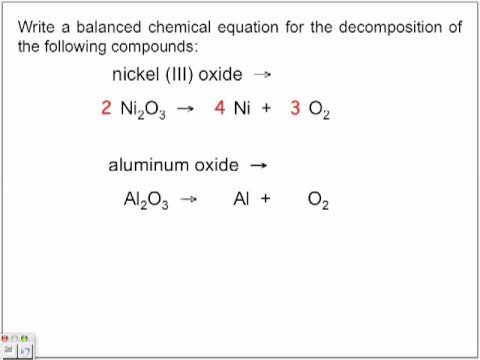
Balanced decomposition reaction. Carbonic acid H2CO3 is an ingredient in soft drinks. The balanced equation for the decomposition of sodium bicarbonate into sodium carbonate carbon dioxide and water is. Write a balanced equation for the decomposition reaction described using the smallest possible integer coefficients.
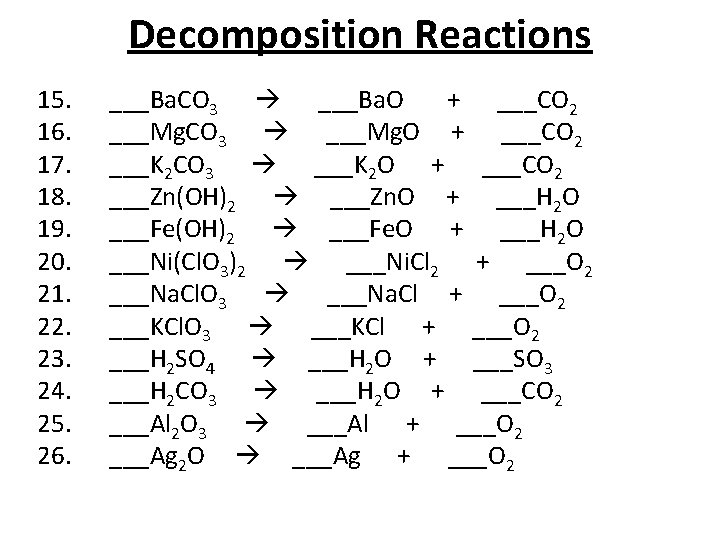
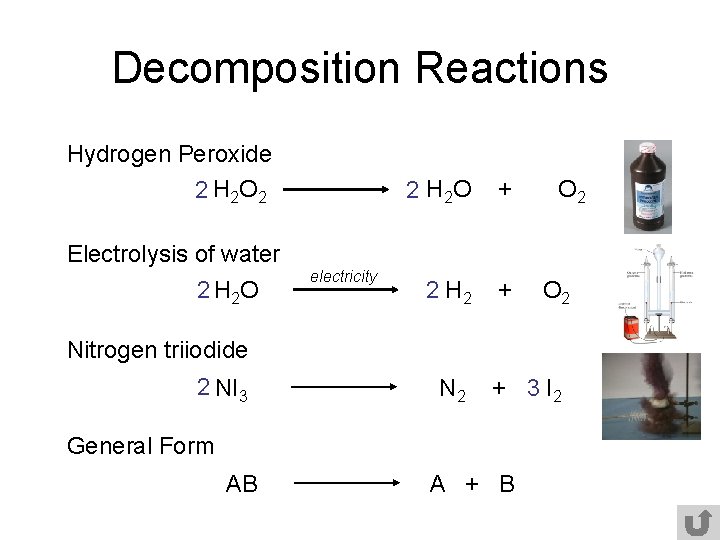
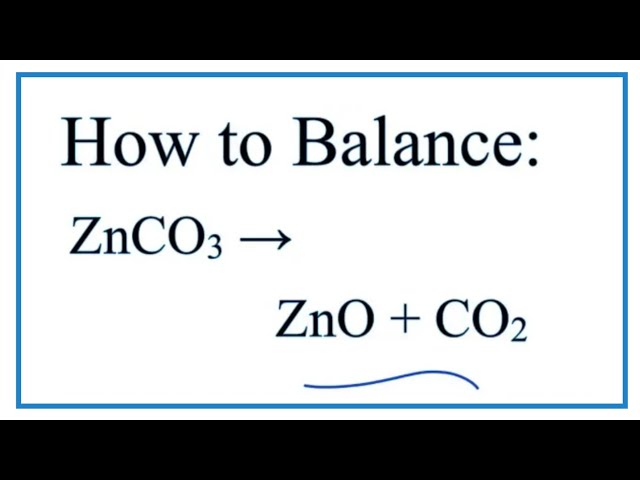
When calcium carbonate decomposes calcium oxide and carbon dioxide are formed. 2AgBrs sunlight 2Ags 2Cl2g - silver bromide decomposes into silver and chlorine in the presence of sunlight. The balanced equation of the decomposition reaction of hydrogen peroxide is that 2H2O2 decomposes into the products 2H2O O2 g.
A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. Water is a binary compound composed of hydrogen and oxygen. The resulting products are water and oxygen gas.
2H2O 2H2 O2. The general form of a decomposition reaction is. Written using generic symbols it is usually shown as.
Write a balanced equation for the decomposition of eqH_2O_2 eq. As a rule of thumb most decomposition reactions are endothermic since energy either in the form of heat electric current or sunlight must be provided in order to break the bonds of the more complex molecule. The breakdown involves formation.
To balance a chemical equation enter an equation of a chemical reaction and press the Balance button. The equation for this reaction is. When an electric current is passed through pure water it decomposes into its elements.