Supreme Khc8h4o4 Naoh Net Ionic Equation

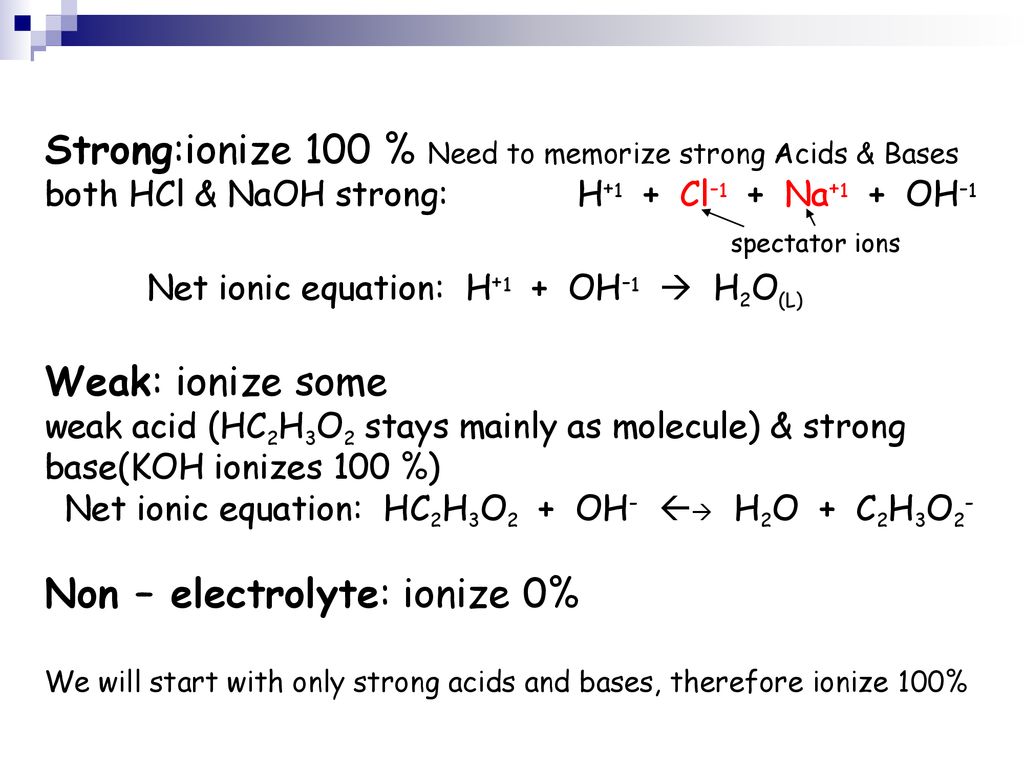
Reactions Involving a Weak Acid or Weak Base.
Khc8h4o4 naoh net ionic equation. Since KHC8H4O4 is aq then it will ionize. HC8H4O4-1aq OH-aq C8H4O4-2 aq H2Ol The reaction can be considered to proceed completely to the right. Hydrogen pthalate ion HC8H4O4- is a weak acid and is produced when KHP abbrevation for KHC8H4O4 is dissolved in water.
The sodium sulfate salt is soluble and so the net ionic reaction is again the same. Different mole ratios occur for other polyprotic acids or bases with multiple hydroxides such as CaOH 2. B if 28 g of 20423 KHP is dissolved in water and titrated with 40 ml of NaOH to the.
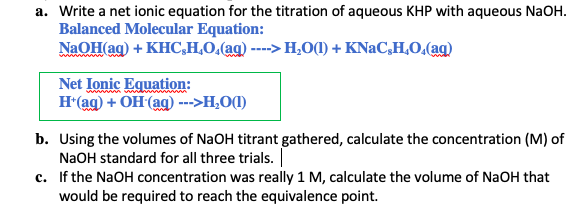
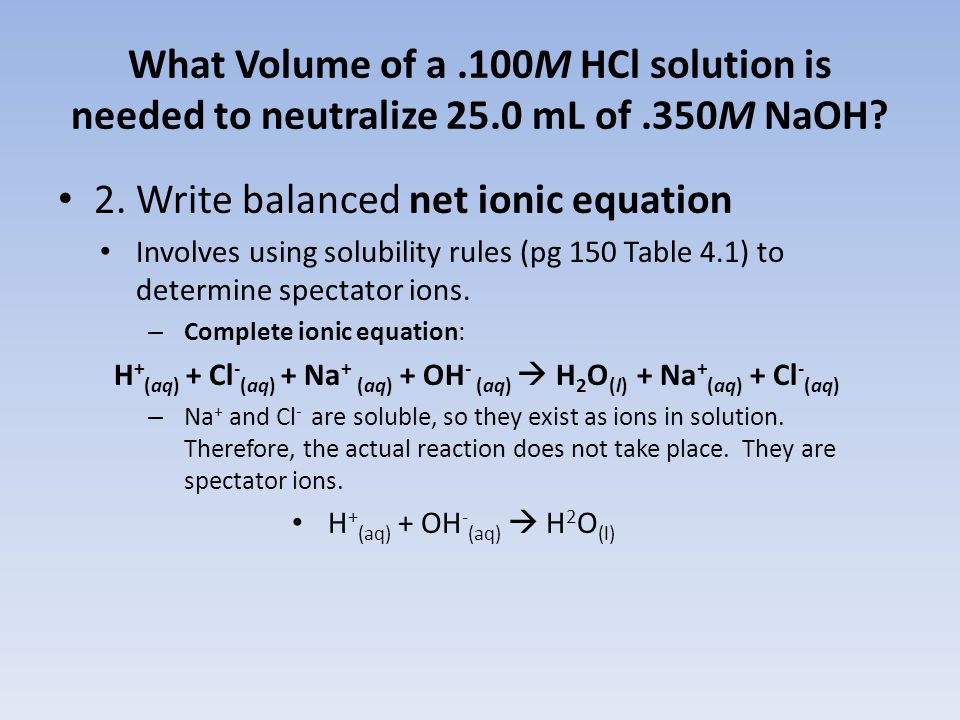
KHC8H4O4 aq NaOH aq KNaC8H4O4 aq H2O l The net ionic equation is. HCl aq NaOH aq NaCl aq H 2O l Since the acid base and salt in this reaction are all strong electrolytes the net ionic equation for this reaction is. Khp is an abbreviation for potassium hydrogen phthalate KHC8H4O4a subtance of known high purity with one acid hydrogen as indicated by the formula.
H aq OH-aq H 2O l. 1 a write balanced molecular and net ionic equations for the neutrilization of NaOH by KHP mol. There are three main steps for writing the net ionic equation for H2SO4 NaOH Na2SO4 H2O Sulfuric acid Sodium hydroxide.
Write the net ionic equation for the unknown titration reaction in this experiment. First we balance the molec. When KHP and NaOH combine a positive hydrogen ion leaves the KHC8H4O4 and a negative hydrogen atom leaves the NaOH.
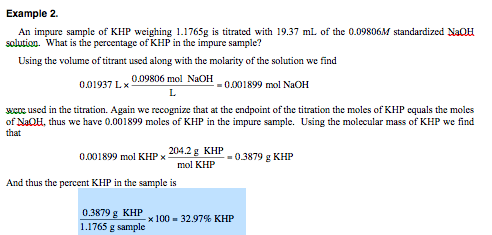
102 g of KHP is dissolved in 1000 mL of solution which is then titrated with NaOH solution of unknown concentration. Strong acid HCl with the strong base NaOH produces the salt sodium chloride and water. Molar mass - gmol weight - g.