Fabulous Propane And Oxygen Balanced Equation

H 2 SO 4 K 4 FeCN 6 KMnO 4.
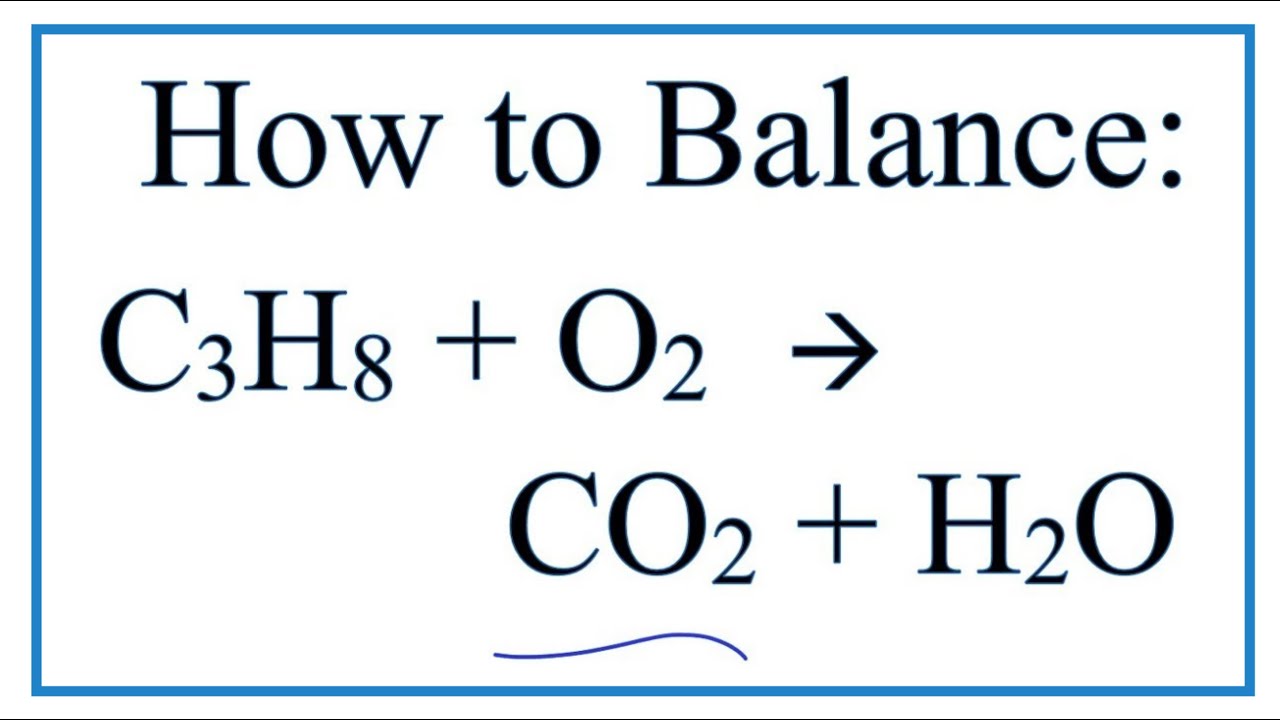
Propane and oxygen balanced equation. If not enough oxygen is present for complete combustion incomplete combustion occurs. G of propane C3H8 is burned in excess oxygen according to the following equation. Oxygen to form gaseous carbon dioxide and gaseous water.
The word equation is. C 8 H 18 O 2. Propane oxygen water carbon dioxide.
Na 2 S 2 O 3 I 2. Propane oxygen Give us feedback about your experience with chemical equation balancer. Since the product side contains 32 41 10 moles of oxygen reactant O2 must be multiplied by 5 to have 10 moles as well.
Propane C3H8 reacts with oxygen O2 to make carbon dioxide CO2 and water H2O. The result of incomplete combustion is once again water vapour carbon dioxide and heat. This is the chemical reaction in which C3H8 or propane burns in air or oxygen to produce carbon dioxide and water.
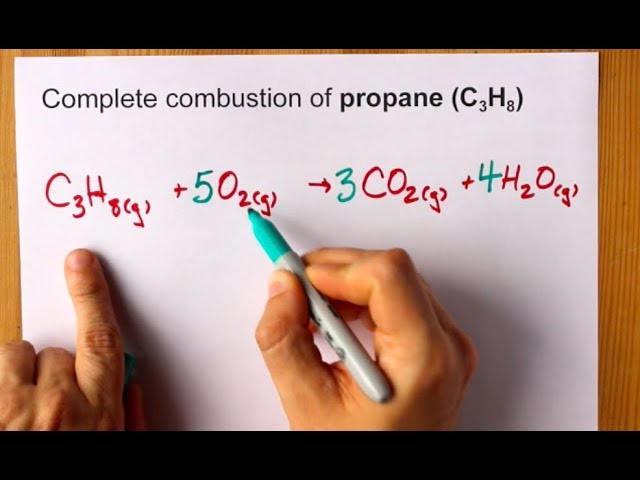
Propane C3H8 burns in oxygen to produce carbon dioxide and water vapor. C subscript 3 H subscript 8 space g space plus space 5 space O subscript 2 space g space rightwards arrow 3 space C O subscript 2 space g space plus space 4 space H subscript 2 O space g How many liters of carbon dioxide are produced at STP when 44 g of C3H8 completely reacts with oxygen. Examples of the chemical equations reagents a complete equation will be suggested.
The balance equation for this reaction is C3H8 5O2 ---- 4H20 3CO2. Propane gas C3He reacts with oxygen according to the balanced equation shown below. C 3 H 8 5 O 2 3 CO 2 4 H 2 O heat.