Great Ammonia Reacts With Hydrogen Chloride To Form Ammonium Chloride

When these two gases are cool enough they react together to form ammonium chloride again.
Ammonia reacts with hydrogen chloride to form ammonium chloride. Elements Compounds and Mixtures. Ammonia and hydrogen chloride react to form solid ammonium chloride. Barium oxide BaO reacts with water to form barium hydroxide.
Write an equation for this reaction. It breaks down when heated forming ammonia and hydrogen chloride. To happen this second step reaction ammonia is required.
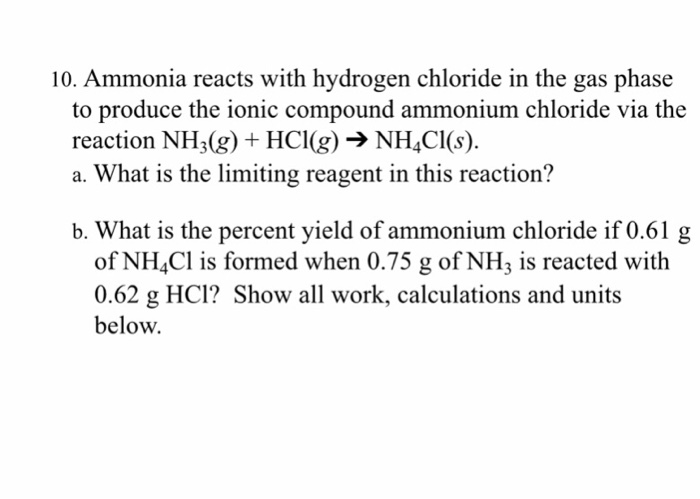
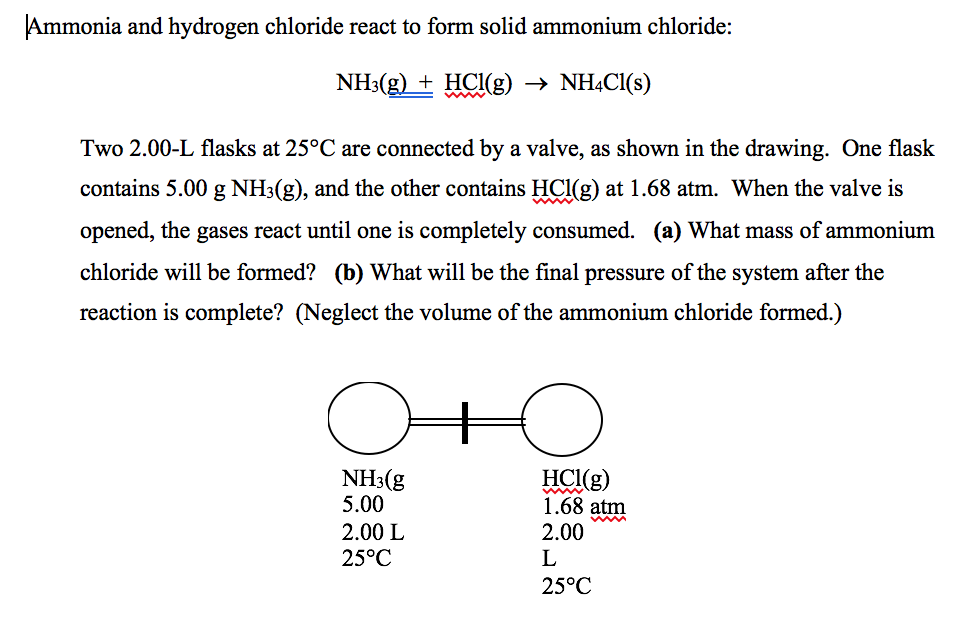
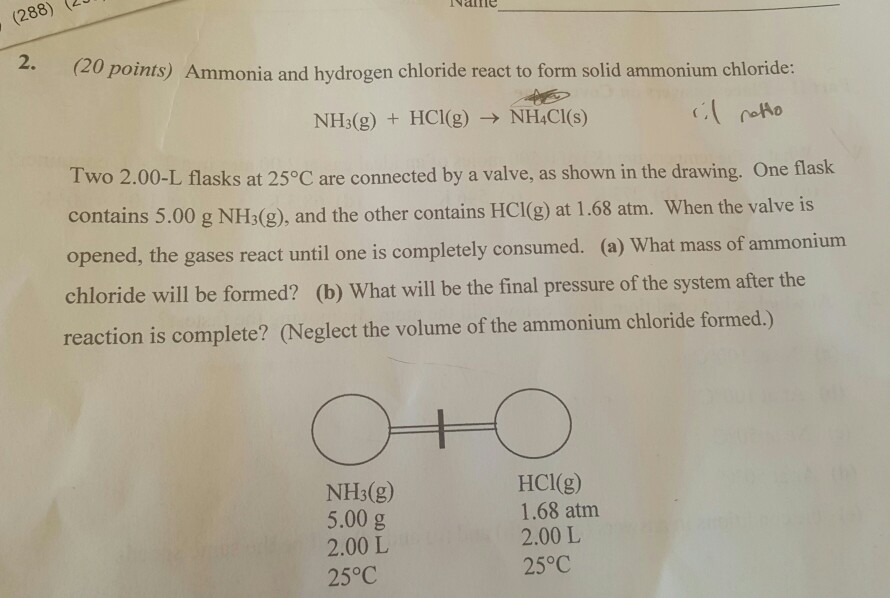
N H 3 g H C l g N H 4 C l s Two 200-L flasks at 25 C are connected by a valve as shown in the drawing on the next page. This reversible reaction can be. Ammonia NH 3 g and hydrogen chloride HCl g react to form solid ammonium chloride NH 4 Cl s.
Researchers have long suspected additional electrons floating around in the high-volume environment could somehow help the ammonia and hydrogen chloride molecules to react. Translate the following statements into chemical equations and then balance them. N H 3 g H C l g N H 4 C l s Two 200 L flasks at 25 C are connected by a valve as shown in the drawing.
One flask contains 500 g of N H 3 g and the other contains 500 g of H C l g. The Diffusion of Hydrogen Chloride and Ammonia Gas through Air to form Ammonium Chloride. Solution Ammonia gas and hydrogen chloride gas react to form the salt ammonium chloride.
Ammonia rapidly reacts with hydrogen chloride making ammonium chloride. NHHS diffuses to another reaction plane 2 at x A where it reacts with the ammoniacal complex of zinc chloride diffusing from the opposite direction to form ZnNHCI NH4HS 3 H 2 0 ZnS - zinc sulfide and ammonium hydroxide. One flask contains 500 g N H 3 g and the other contains 500 g H C l g.