Cool General Equation Of Decomposition Reaction
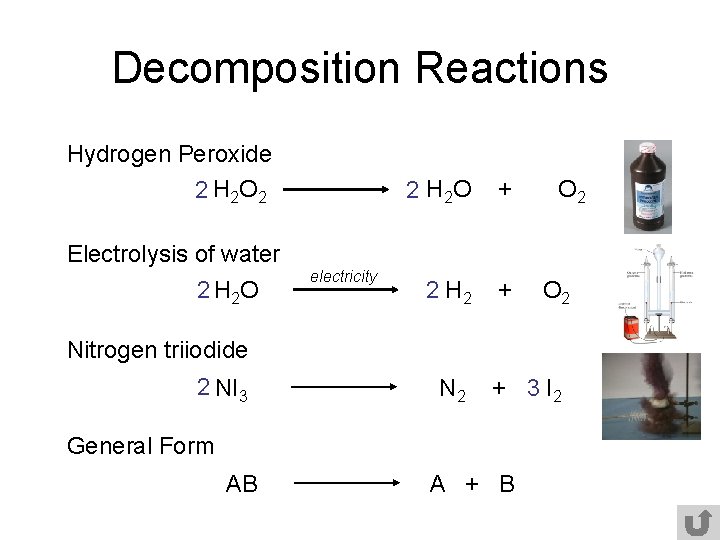
2H2O 2H2 O2.
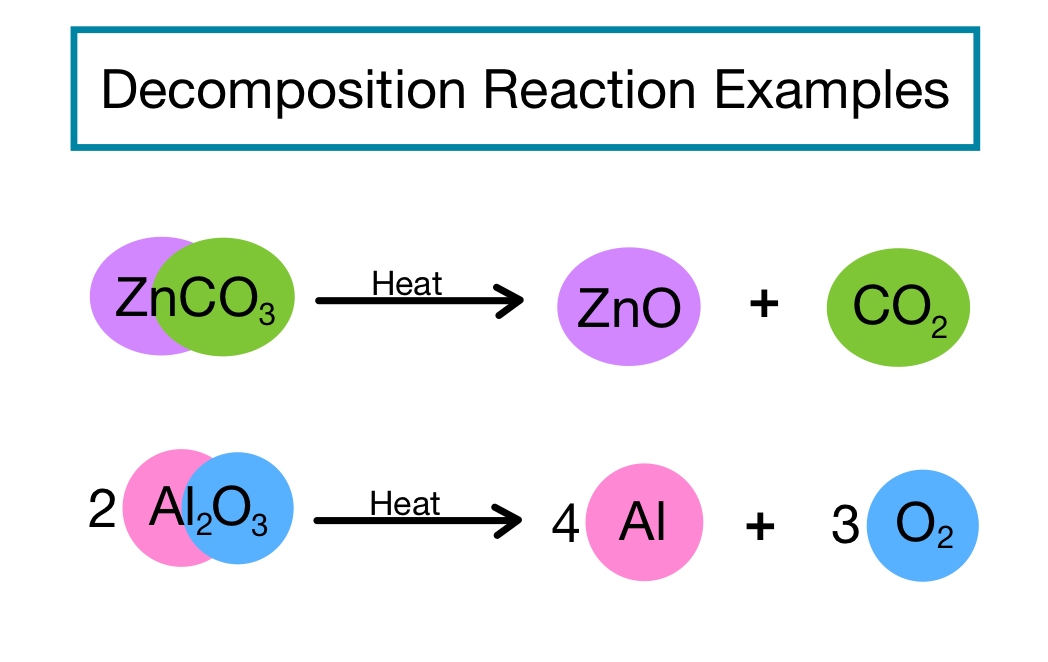
General equation of decomposition reaction. Examples of decomposition reactions include the breakdown of hydrogen peroxide to water and oxygen and the breakdown of water to hydrogen and oxygen. Written using generic symbols it is usually shown as. Then the carbonic acid spontaneously decomposes to produce carbon dioxide and water.
When an electric current is passed through an aqueous solution of a compound it may result in an electrolytic decomposition reaction. During decomposition one compound splits apart into two or more pieces. The general pattern of a decomposition reaction is.
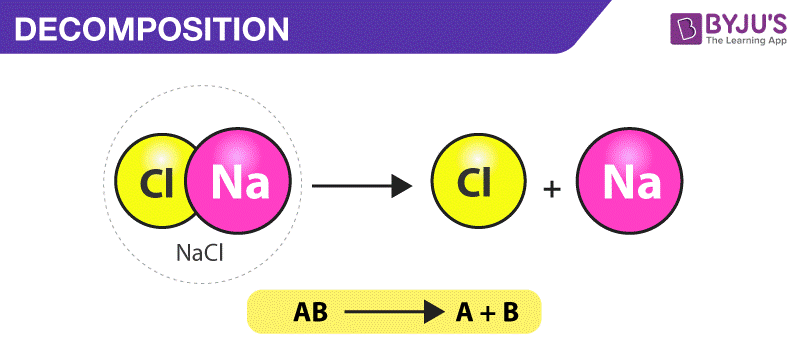
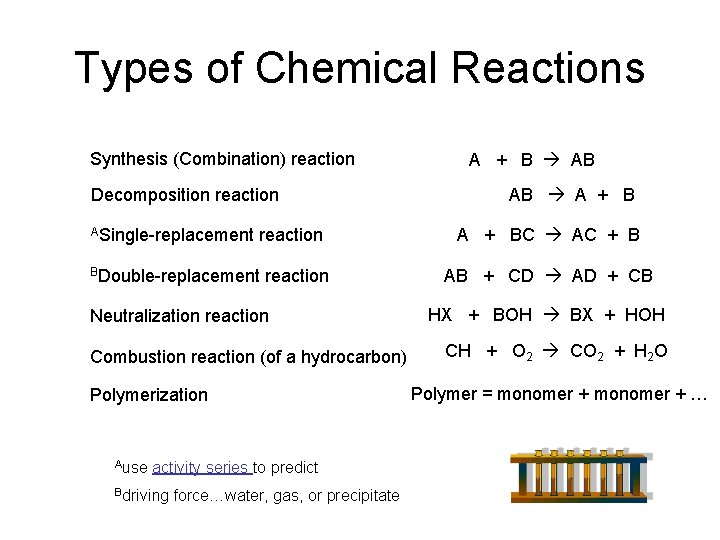
AB -- A. This tutorial focuses on the decomposition of a binary compound. How do you classify a decomposition reaction.
The topic Decomposition Reaction is covered in the unit 1 chapter 1 of NCERT Class 10 Science. The official meaning of decomposition is a little bit more specific and means a reaction in which one chemical splits into two or more chemicals like this. Describes the basics of decomposition reactions how to identify them predict the products and balance the chemical equation.
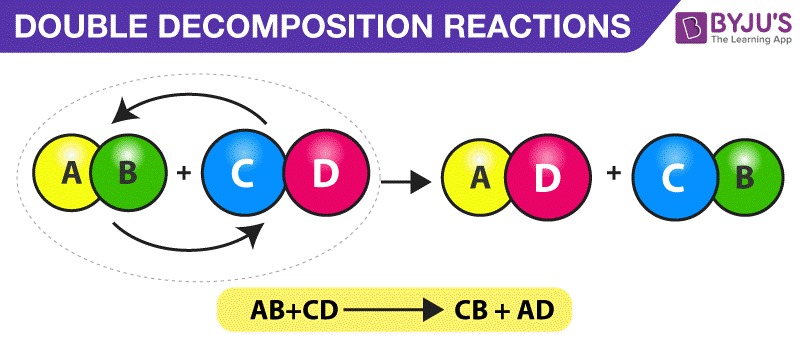
AB A B. For example copper carbonate breaks down easily when it is heated. Iii Double Decomposition reactions The most common example of this type of reaction is the reaction between an acid and a carbonate or hydrogencarbonate bicarbonate 9 Initially the acid reacts with the carbonate or hydrogencarbonate to produce a salt and carbonic acid H 2 CO 3.
Decomposition reactions In a decomposition reaction a chemical substance is broken down into simpler substances. Two examples are also shown d. This can berepresented by the general equation.