Impressive General Equation For Incomplete Combustion
The formula for ethanol is given by.
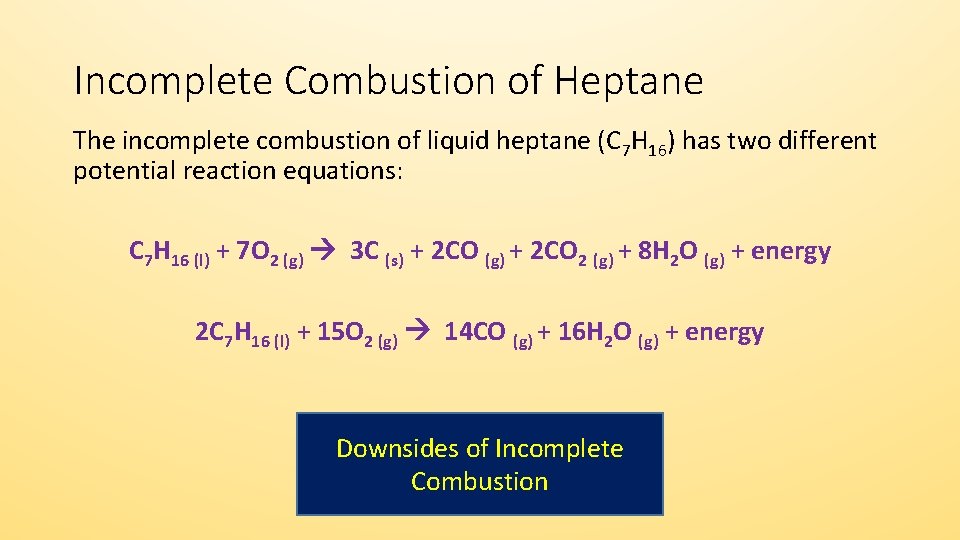
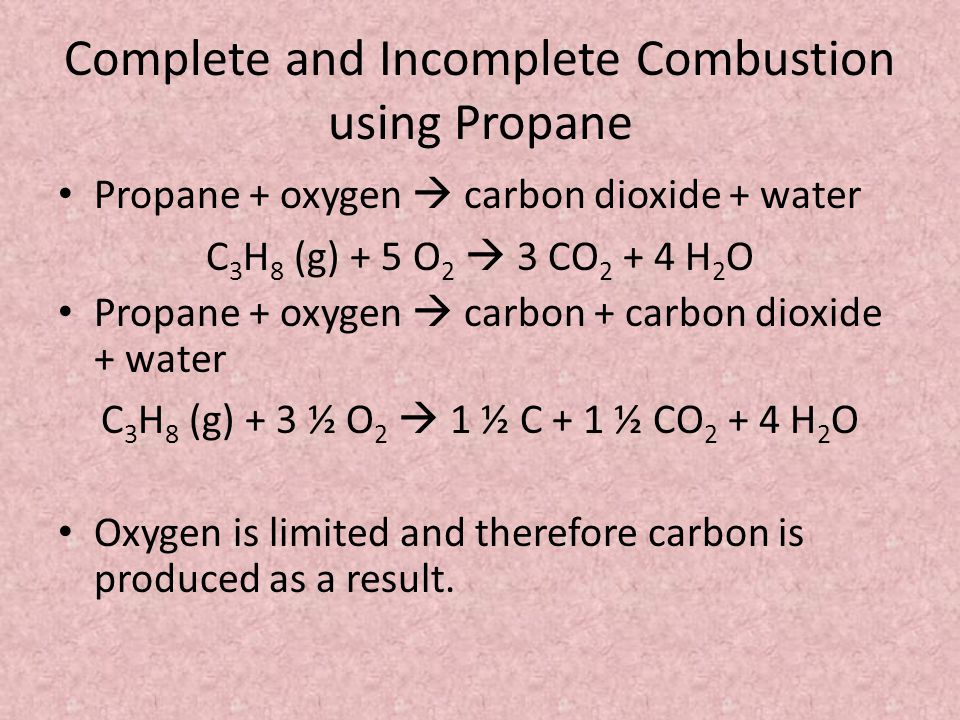
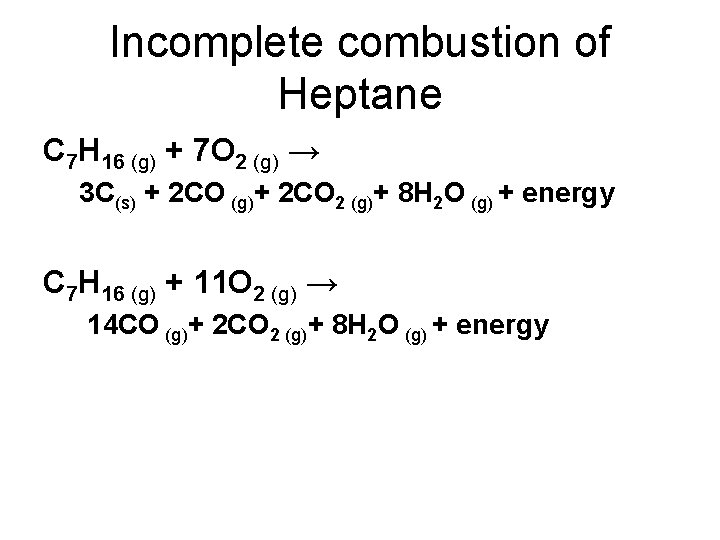
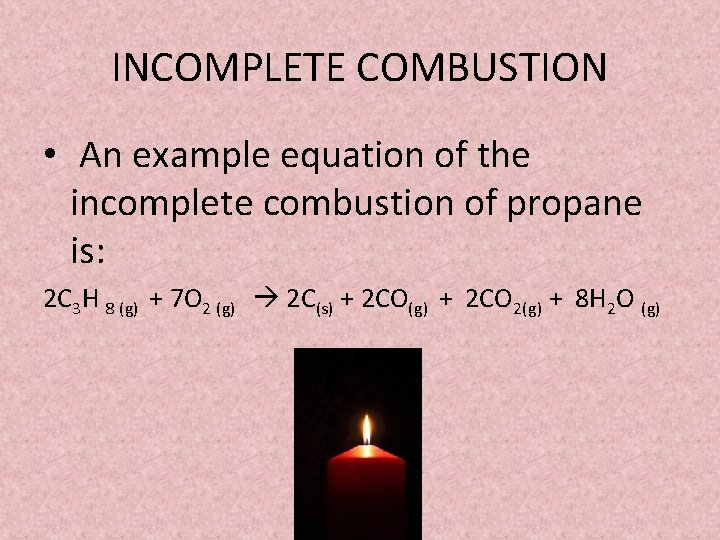
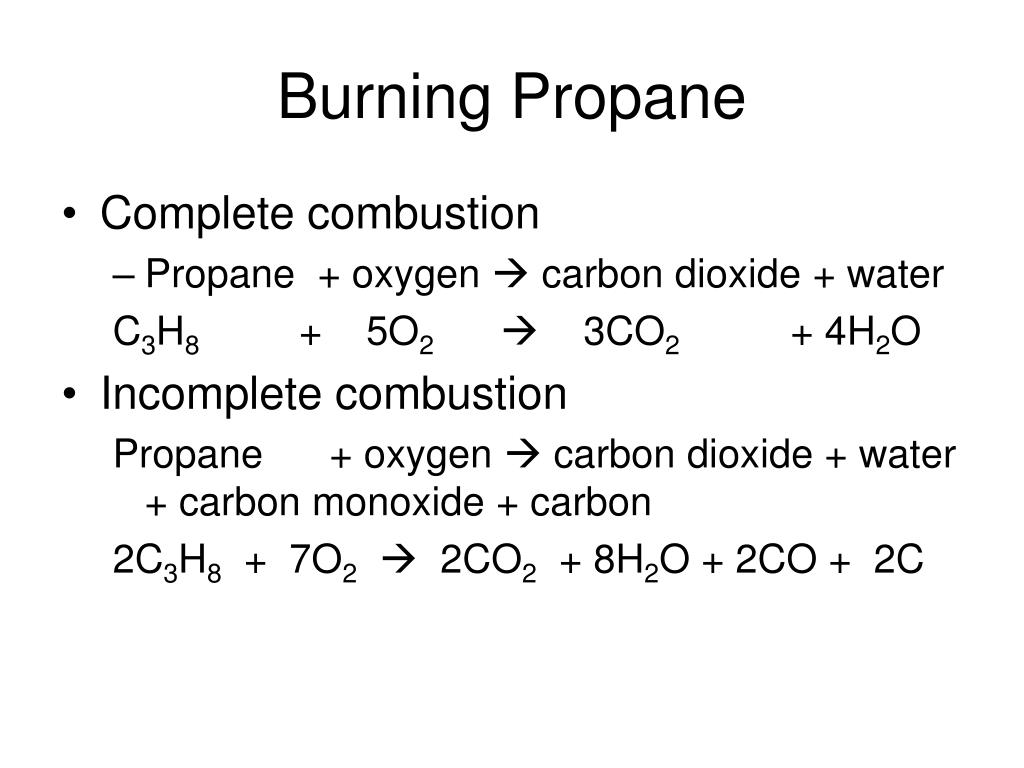
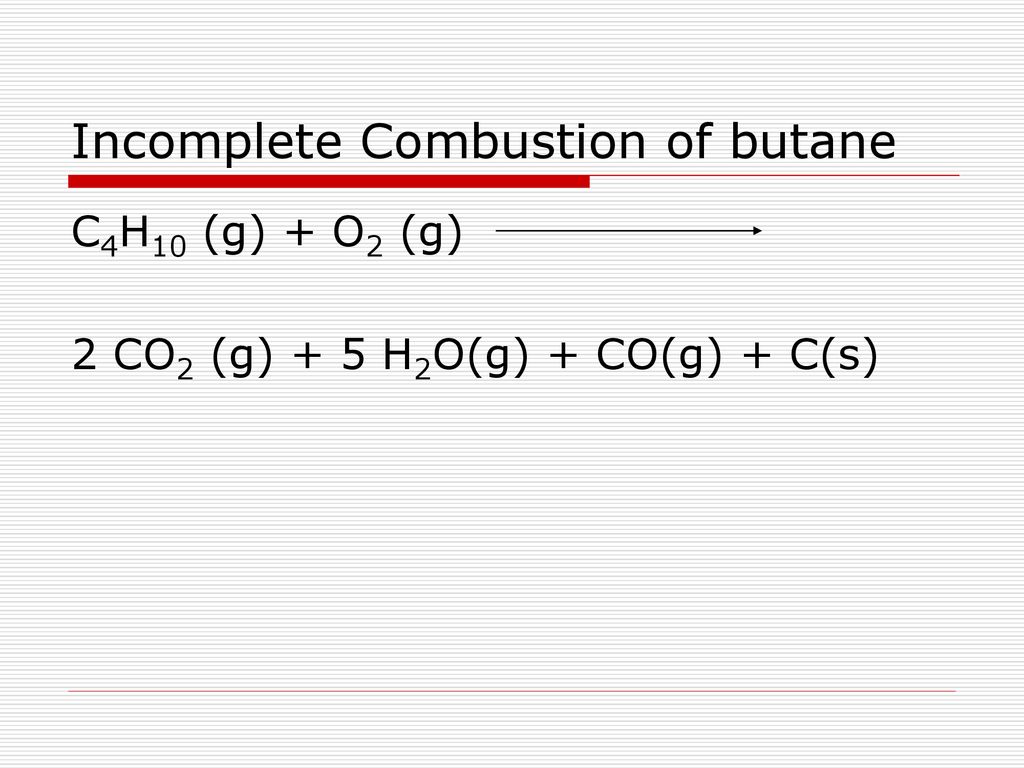
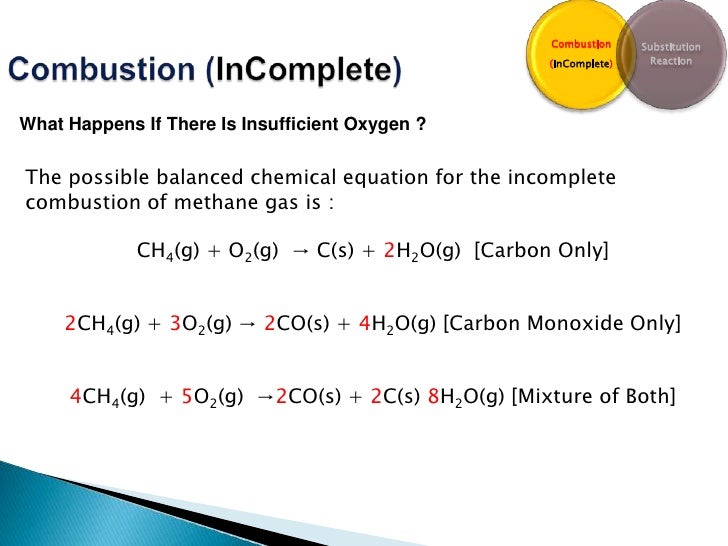
General equation for incomplete combustion. Write the balanced equations for the complete incomplete combustion of 2 pentene. Water is still produced but carbon monoxide and carbon are produced instead of carbon dioxide. Oxygen gas is the limiting reagent hydrocarbon is the reactant in excess The incomplete combustion of a hydrocarbon usually produces a sooty flame due to the presence of carbon C or soot as a product of the incomplete combustion.
808 Explain why the incomplete combustion of hydrocarbons can produce carbon and carbon monoxide. General equation for incomplete combustion reaction follows. But the amount of energy released from this combustion is comparatively low.
Water as a product is obtained in complete combustion as well. In general for incomplete combustion. So if in a chemical reaction carbon monoxide or carbon are produced it will result in incomplete combustion.
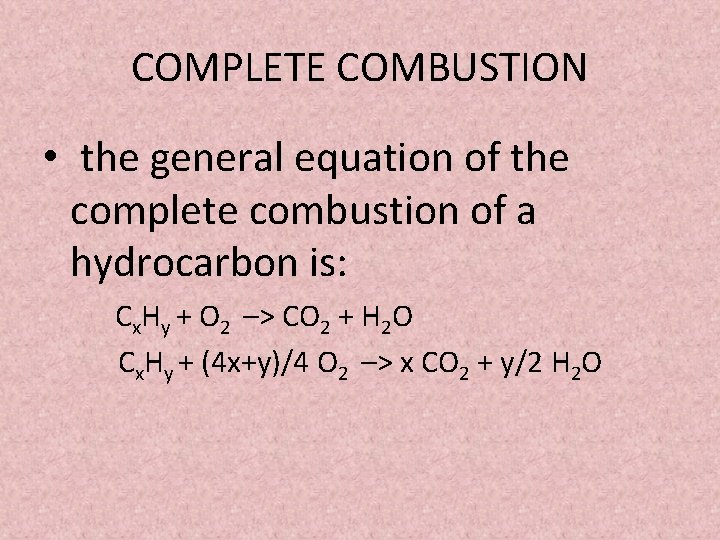
Ethanol is a fuel source in an alcohol lamp. Methane oxygen gas solid carbon water vapour. Complete combustion given sufficient oxygen of any hydrocarbon produces carbon dioxide and water.
Thus the correct answer is CO. Click to see full answer. For example with alkanes the ones with an even number.
The air hole is closed. In general most elements in a compound that is combusted will form oxides but you wont be able to say for sure how much of each oxide will be produced CO or CO 2 SO 2 or SO 3 etc. Recognizing the characteristics and balancing incomplete combustion reactions.