Formidable Decomposition Of Ammonium Carbonate

It decomposes in hot water 58 C releasing ammonia and carbon dioxide.
Decomposition of ammonium carbonate. B Observations on heating NH2CO. Ammonium carbonate is a white solid with a strong odor of ammonia soluble in water. Because of the ease of decomposition and the penetrating odor of ammonia ammonium carbonate can be used as smelling salts.
Ammonium acetate ammonium carbonate ammonium nitrate ammonium oxalate and ammonium phosphate. Ammonium bicarbonate is an inorganic compound with formula NH 4HCO 3 simplified to NH 5 CO 3The compound has many names reflecting its long history. It has a density of 150 gcm 3.
Chemically speaking it is the bicarbonate salt of the ammonium ion. The key feature of this method is a pH gradient germinated by the diffusion of NH 3 and CO 2 vapors in the metal salts solutions from solid NH 4 2 CO 3 in a closed environment. AMMONIUM CARBONATE decomposes when heated to give gaseous ammonia and gaseous carbon dioxide.
Decomposition of sodium hypochlorite takes place within a few seconds with the following salts. The ammonium carbonate is an inorganic salt nitrogen ammoniacal specifically the chemical formula NH 4 2 CO 3. Solid ammonium carbonate NH 4 2 CO 3 decomposes at room temperature to form gaseous ammonia carbon dioxide and water.
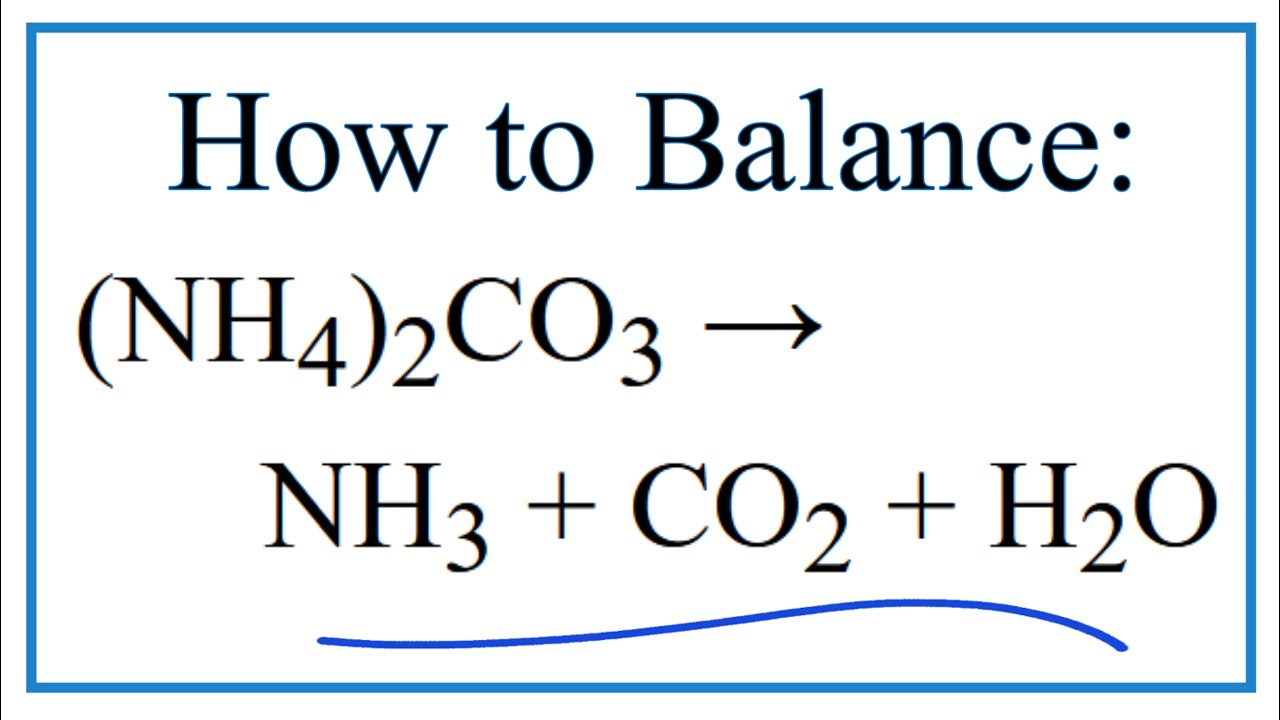
The following balanced equation illustrates the decomposition reaction of ammonium carbonate when it is heated. The decomposition of ammonium carbonate at room temperature is demonstrated by the equation NH42CO3. In the formula NH42CO3 represents the ammonium carbonate on the reactant side of the equation while NH4HCO3 and NH3 represent ammonium bicarbonate and ammonia on the product side respectively.
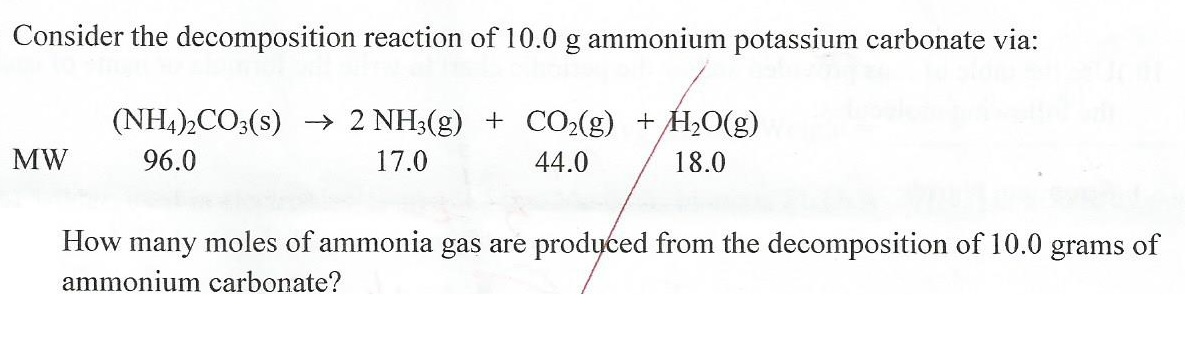
B A 1250-g sample of ammonium carbonate is completely decomposed upon. Ammonium carbonate is available as bakers ammonia in some food stores though most will sell only ammonium bicarbonate. 14TH Edition Quincy MA 2010.