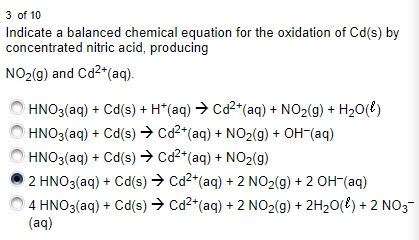Formidable Balanced Equation Of Nitric Acid

A Chemical equation is.
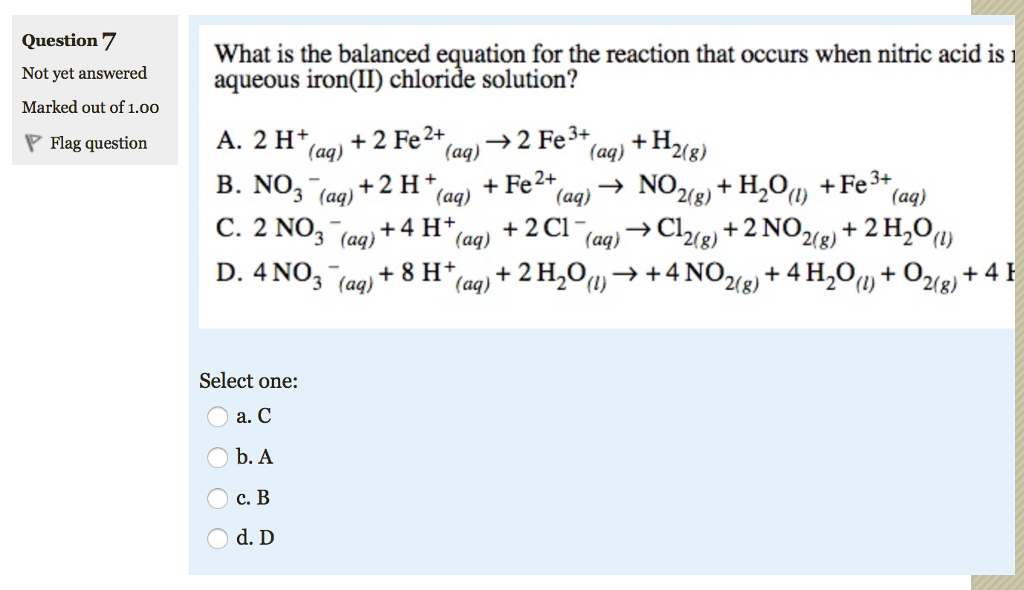
Balanced equation of nitric acid. Sulphur Nitric Acid Sulphuric Acid Nitrogen Dioxide Water. How to Balance Mg OH2 HNO3 Mg NO32 H2O Magnesium hydroxide Nitric acid When balancing chemical equations our goal is to have the same number of each type of atom on both sides of the equation. The Aluminum ion instead will react with water releasing a Hydrogen ion.
When Aluminum Nitrate is dissolved in water the salt first dissociates in water. This acid dissociates in water to yield hydrogen ions and nitrate ions NO 3 - in a reaction analagous to the dissociation of carbonic acid shown in Equation 2 again lowering the pH of the solution. B Concentrated hydrochloric acid cannot replace Conc.
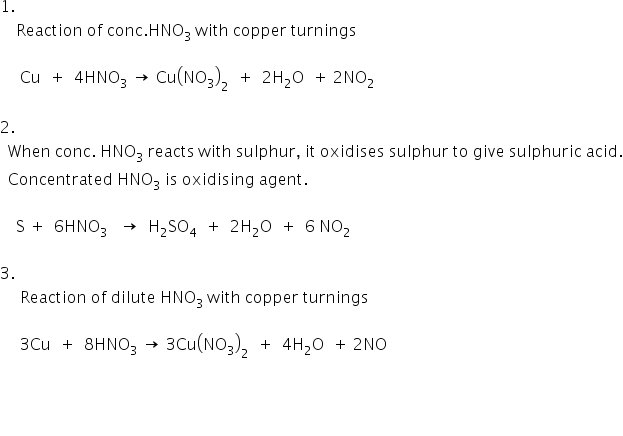
The nitric acid is ionic compound which is soluble in the water. Sulphuric acid for the preparation of nitric acid because hydrochloric acid is volatile acid and hence nitric acid vapours will carry HCl vapours. The balanced chemical equation for the laboratory preparation of nitric acid is as follows.
Identify all of the phases in your answer. Write the Balanced Chemical Equations of the Following Reactions. In this video well balance the equation Potassium hydroxide Nitric Acid and provide the correct coefficients for each compoundTo balance KOH HNO3 KN.
Only change the coefficients these are the numbers in front substances. Type of Chemical Reaction. Write balanced molecular equation for the reaction between nitric acid and calcium hydroxide Express your answer as a chemical equation.
Balanced equation of calcium and dilute nitric acid reaction Ca s 2HNO 3 aq Ca NO 3 2 aq H 2 g Calcium and concentrated nitric acid reaction When nitric acid concentration is considerably high it behaves as an oxidizing acid. This chemical equation is a bit challenging to balance because you need to carefully keep track of the O atoms as you work. Never change the subscripts the small numbers after elements.