Glory Baking Soda And Sulfuric Acid Equation

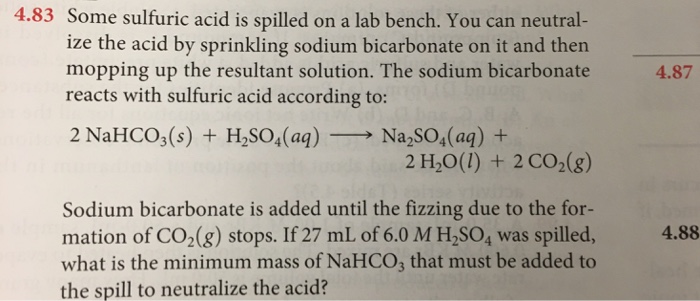
Some sulfuric acid is spilled on a lab bench.
Baking soda and sulfuric acid equation. This will neutralize light acids like vinegar or even strong dangerous acids like muriatic and sulphuric acids. Neutralize spill with sodium bicarbonatebaking soda 2. Be careful not to over- neutralize 4.
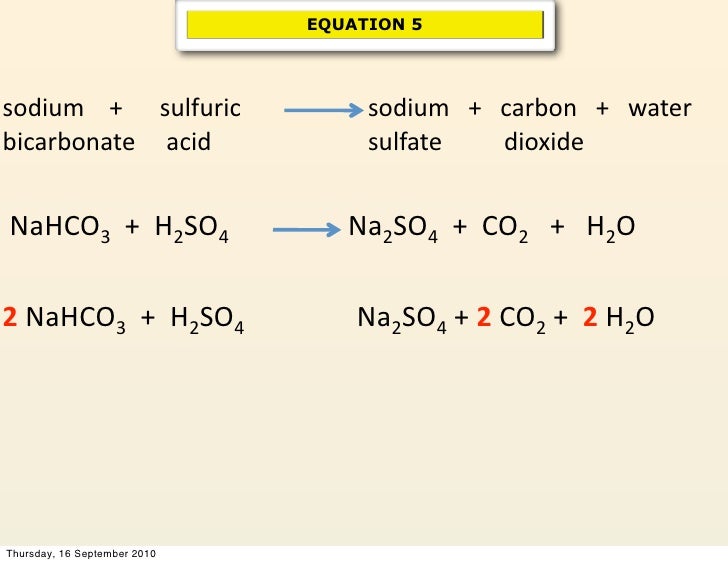
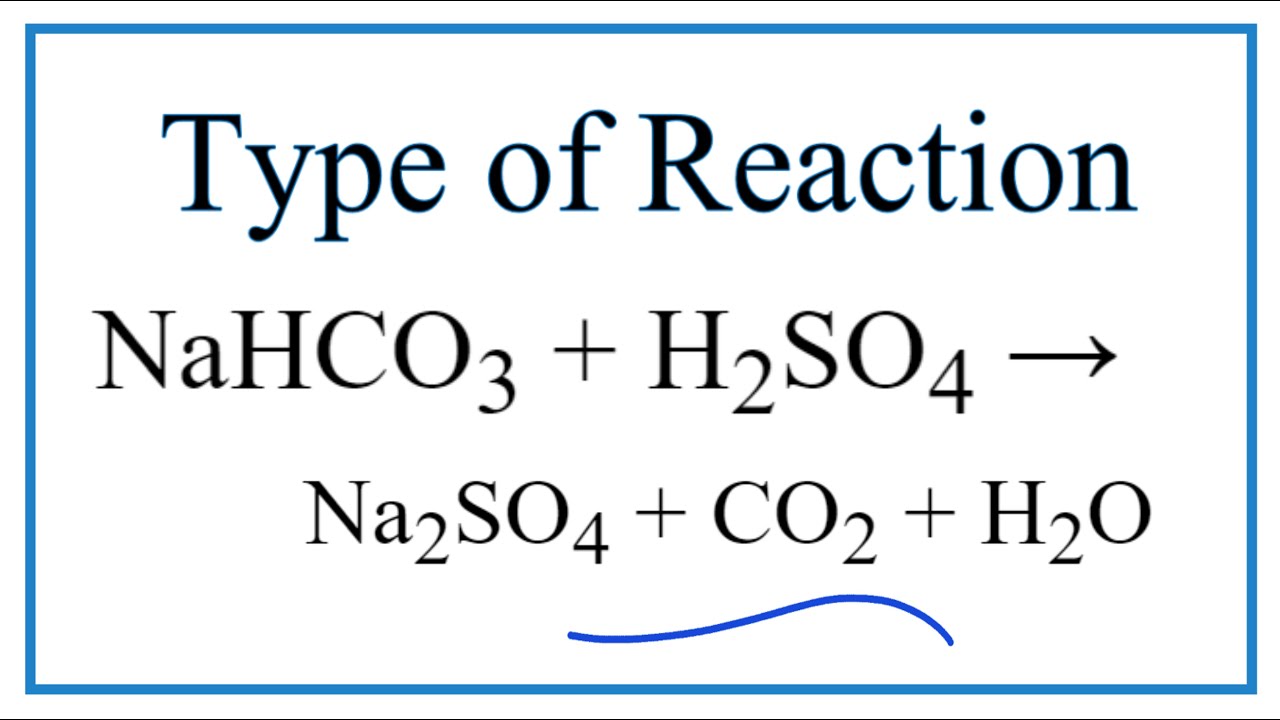
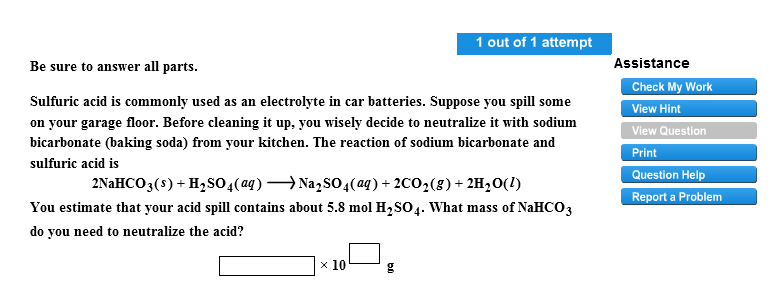
The sodium bicarbonate reacts with sulfuric acid in the following way. Douse the entire affected area with the baking soda sodium bicarbonate NaHCO3 to neutralize the acid. The iron vessel is filled with a sodium bicarbonate solution.
Just like the vinegar and the baking soda when sulfuric acid is mixed with a base the two will neutralize each other. Baking soda is sodium bicarbonate NaHCO3 and sulfuric acid is H2SO4. 2NaHCO3 H2SO4 Na2SO4 2H2O 2CO2 So this means 2 moles of sodium bicarbonate is needed to neutralise 1 mole of sulfuric acid.
It consists of a strong iron vessel with a side discharge nozzle. Wait until bubblingfizzing has stopped 3. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
Baking soda is basic in nature and bitter in flavor. It is commonly used to neutralize unwanted acid solutions or acid spills. It is the most efficient house-hold fire extinguisher.
Baking soda is. When using a neutralizing spill kit the kits are buffered and will not have a bubbling action. This kind of reaction is called a neutralization reaction.