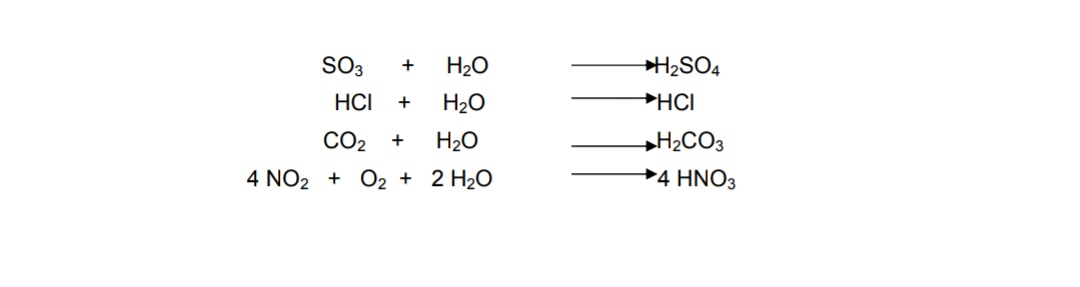Fun Acid Rain Reaction Equation

H2O R2 COgÛCO H 22 2O.
Acid rain reaction equation. Acid rain falling over regions with alkaline soils or rocks is quickly neutralized by reactions such as taking place once the acid has deposited to the surface. The term acid rain is actually somewhat misleading because even pure rainwater collected in areas remote from civilization is slightly acidic pH 56 due to dissolved carbon dioxide which reacts with water to give carbonic acid a weak acid. Sources of Acid Rain Acid rain is caused by a chemical reaction that begins when compounds like sulfur dioxide and nitrogen oxides are released into the air.
Because surface waters are in equilibrium with atmospheric carbon dioxide there is a constant concentration of carbonic acid H 2 CO 3 in the water. Sulfur dioxide and nitrogen oxides dissolve very easily in. Metals like iron and calcium carbonate react with the acid in the rain slowly as follows.
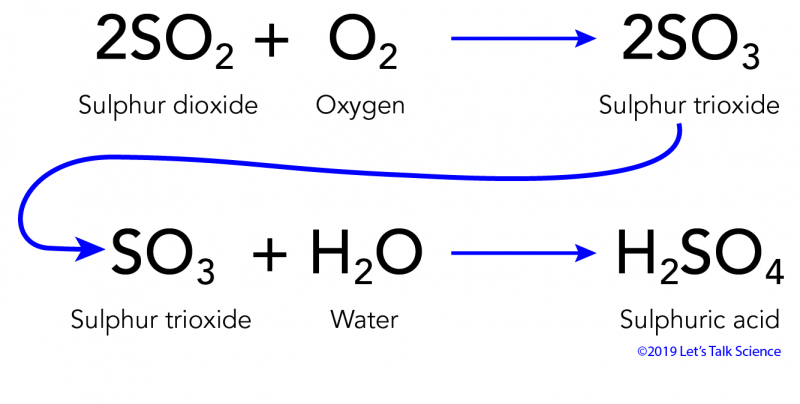
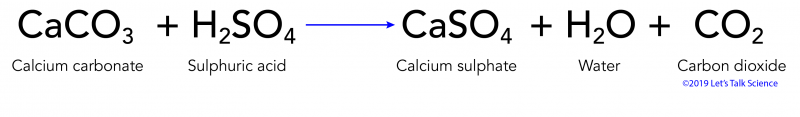
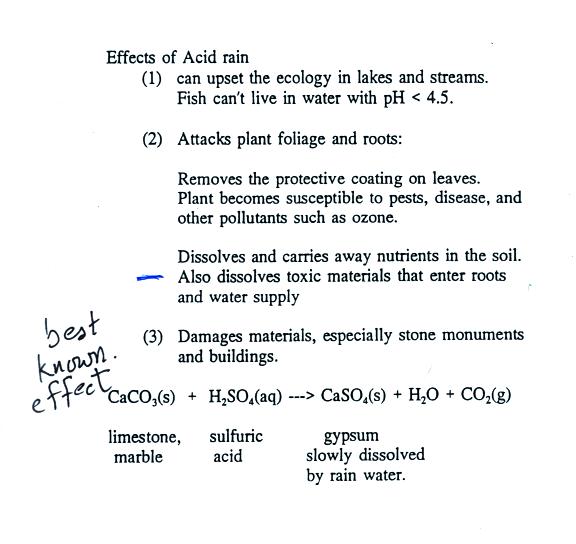
It equilibrates withatmospheric CO2 a weak acid following the reactions presented inchapter 6. Acid rain is caused by emissions of sulphur dioxide and nitrogen oxide which react with the water molecules in the atmosphere to produce acids. When rain falls from the sky onto a limestone CaCO 3 statue a neutralization reaction occurs between sulphuric acid and calcium carbonate.
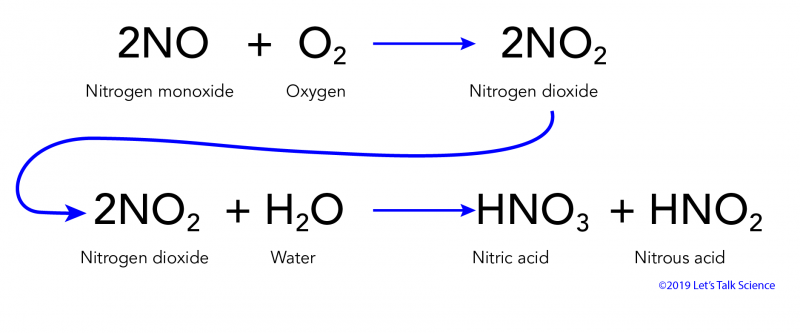
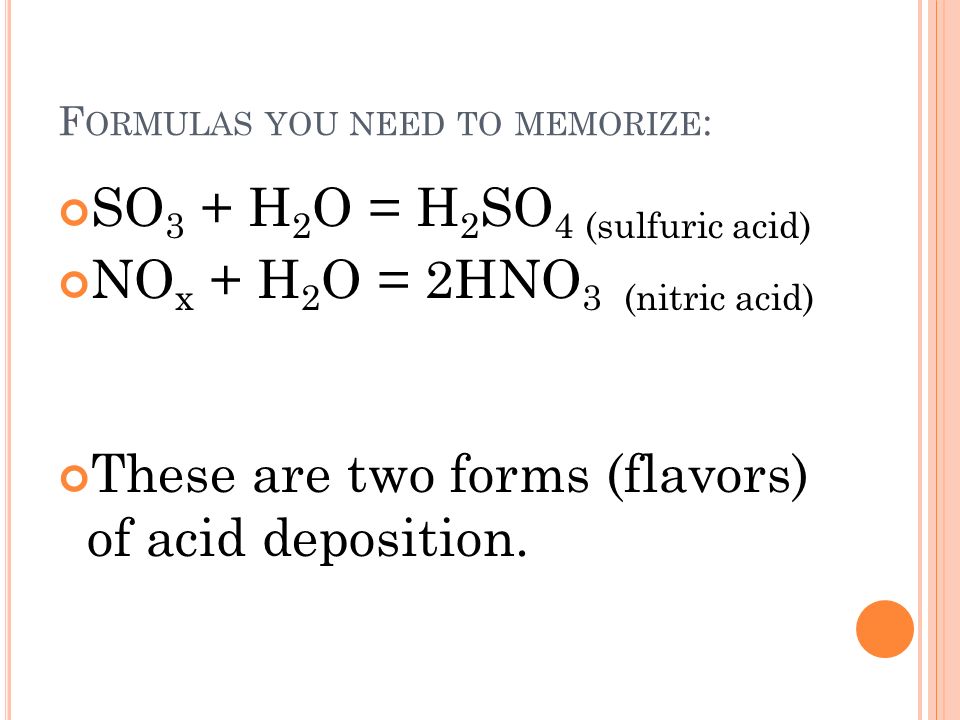
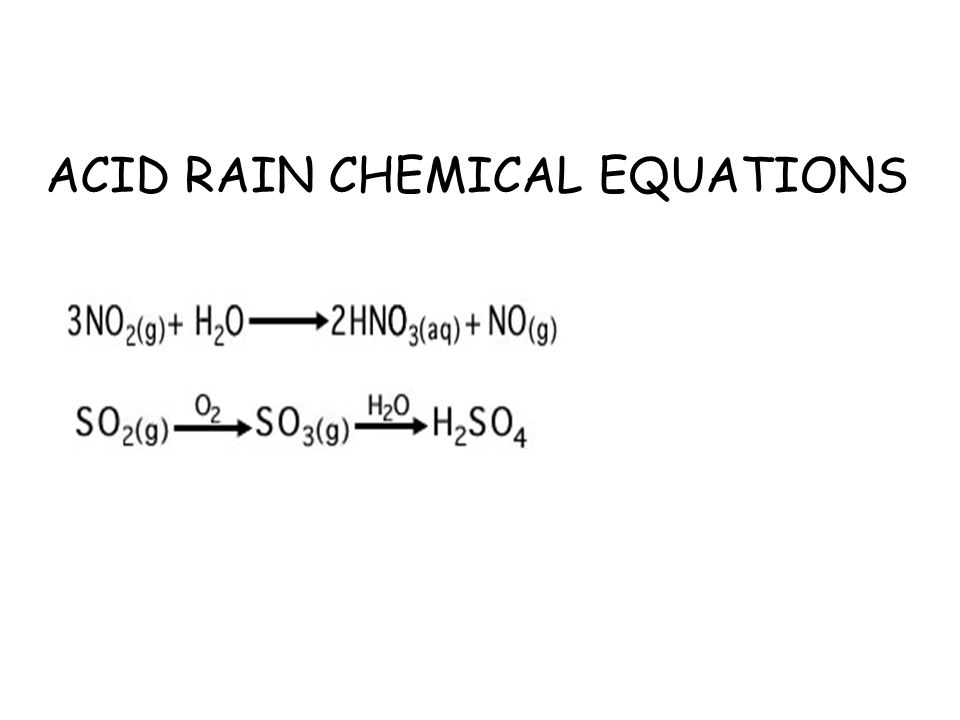
He defines buffers and their compositions talks about carbonate buffering systems in n. The reaction equation for acid rain produced from nitrogen oxides is. 2NO 2 g H 2 O l HNO 3 aq HNO 2 g Acidic rain is mainly caused by atmospheric pollutants of sulfur dioxide and nitrogen oxides.
Part of the country. Acid rain refers to any kind of precipitation that transports nitrogen and sulfur compounds to the Earths surface. The chemical formula of acidic rain is dependent upon the type of acids present.
Limestone is one familiar form of calcium carbonate. Acidic rain is a complex mixture of nitrous nitric sulfurous and sulfuric acids which all combine to lower the pH. This reaction creates calcium sulfate CaSO 4.