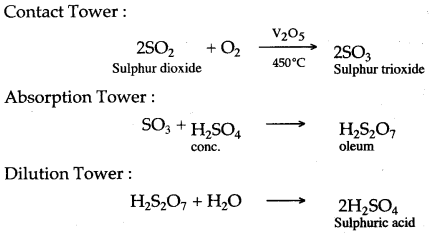
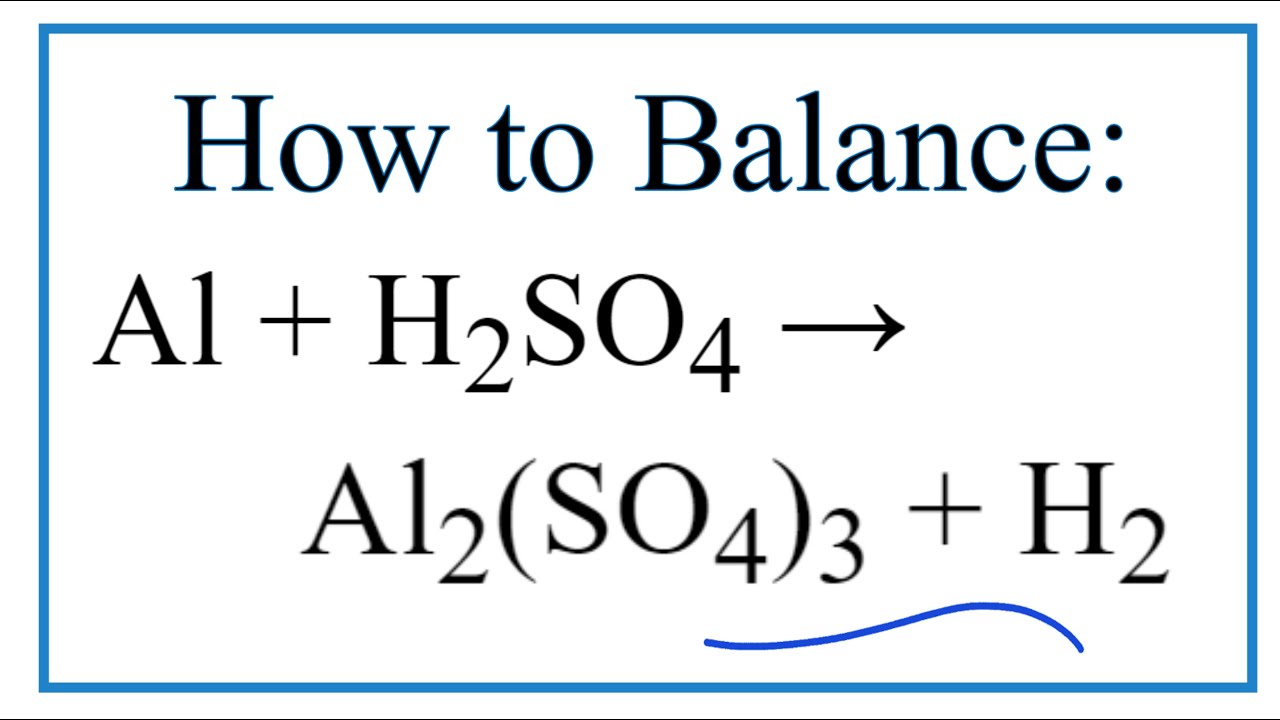
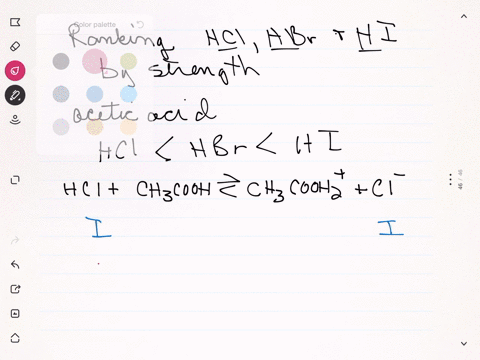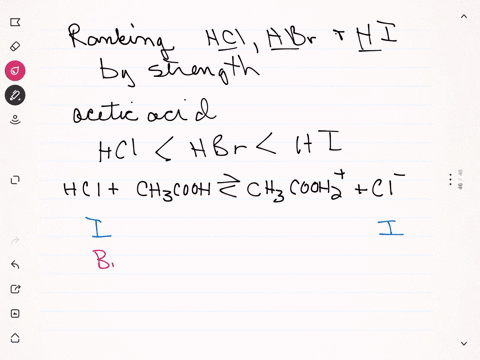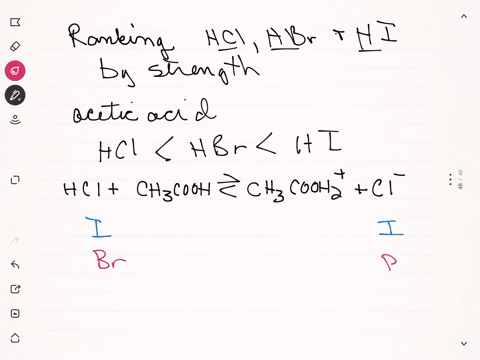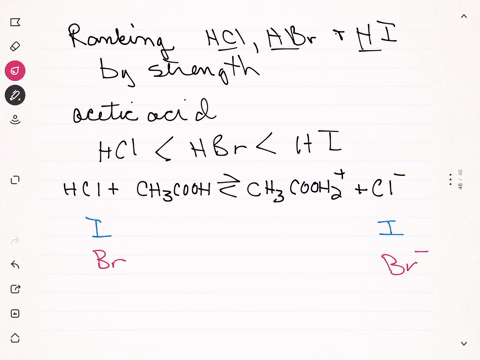First Class Sulfuric Acid Plus Ammonia

There should be an eyewash and safety.
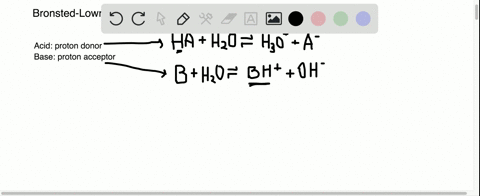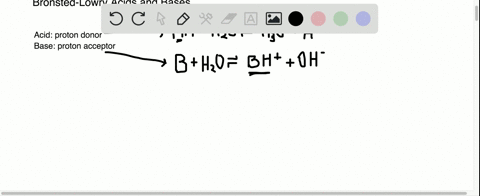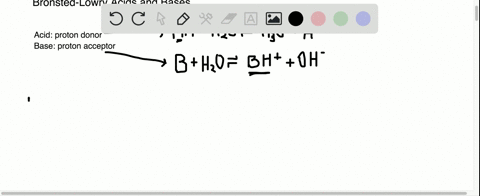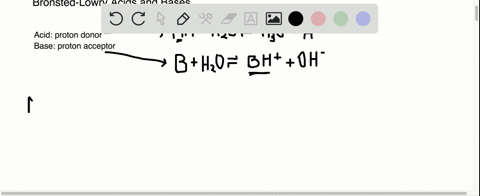
Sulfuric acid plus ammonia. FIRST NEXT If you mix a solution of 0000150M sulfuric acid what will the pH be. Ammonia react with sulfuric acid to produce ammonium sulfate. If your lab has acid or bleach baths discuss this incident in your lab group meeting.
According to safety data sheets Super 8 is a mixture of 810 sodium hypochlorite in water stronger than household bleach. Ammonia reacts with sulfuric acid to produce ammonium sulfate and water. It is a stable acid that works effectively in changing ammonia NH3 into ammonium NH4 which is a plant available N-form that does not evaporate.
2 NH 3 H 2 SO 4 NH 4 2 SO 4 A mixture of ammonia gas and water vapor is introduced into a reactor that contains a saturated solution of ammonium sulfate and about 2 to 4 of free sulfuric acid at 60 C. 2NH 3 H 2 SO 4 - NH 42SO 4. For work with acids bases or bleach have a written SOP that includes protective clothing and emergency procedures for an accident.
2NH3 H2SO4 -. Ammonia NH3 liquid ammonia but rare Concentrated Amonia Solution NH4OH. The advantages of using sulfuric acid are as follows.
Further addition of ammonia causes the copper ion to go back into solution as a deep blue ammonia complex. Now of course as a weak base there is SOME ammonium ion in solution according to the following equilibrium. In the case of Ammonia Scrubbers dilute sulfuric acid H2SO4 is typically used to neutralize the ammonia.
Your answer should be rounded to two decimal places In a solution of 1 liter of hydroiodic acid where you have 000625 moles of HI dissolved what is concentration of H3O ions. There are two reactions that can happen depending on whether the H2SO4 is dilute or concentratedFor Cu. 15molL-1 with respect to ammonia.