Great Potassium With Cold Water Equation

Potassium metals react vigorously or even explosively with cold water.
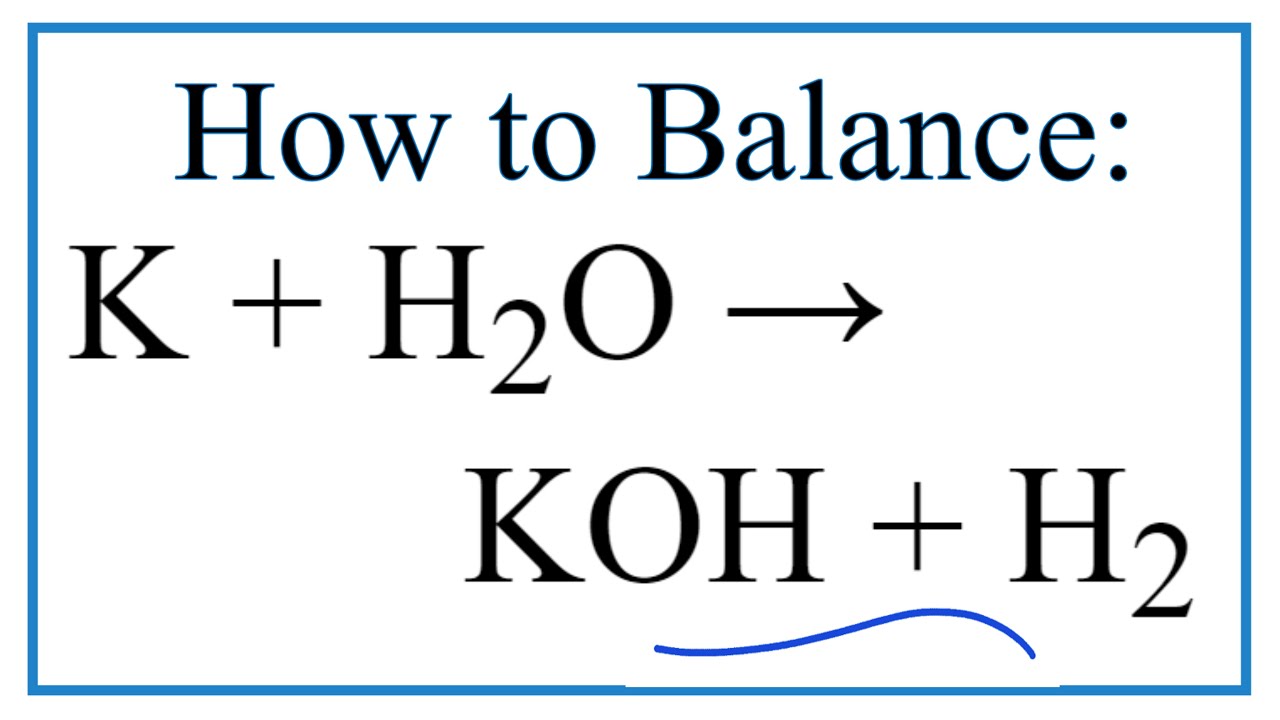
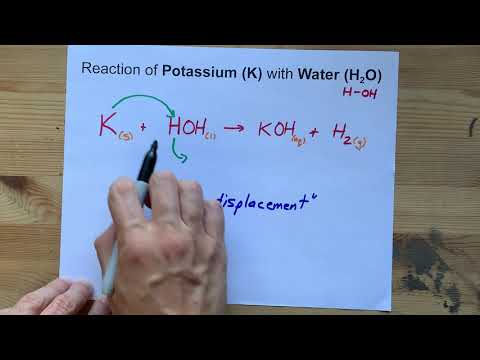
Potassium with cold water equation. General Method Reactions of Active Metals - Cold Water Potassium- K H2o _____ _____ G CISCE ICSE Class 9. The reaction is exothermic. 2K s 2H2O l 2KOH aq H2g heat.
Potassium is a metal with a soft texture and it has a silvery appearance when cut which becomes tarnished as it oxidizes in air. Rubidium Water Rubidium hydroxide Hydrogen. Padi Elearning Login Problem KNH 2 H 2 O --- K NH 3 OH-2.
During the process much heat is generated that the hydrogen gas generated caught on fire and rapidly burns. When potassium reacts with water it catches fire generating a purple glow. 2K 2H2O 2KOH H2.
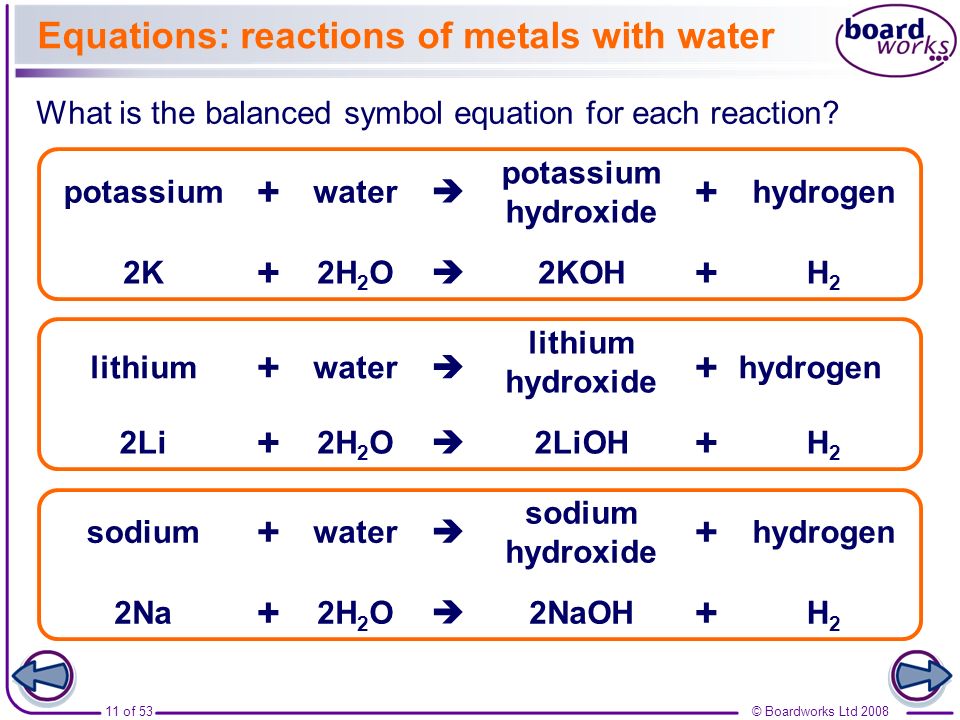
All of these metals react vigorously or even explosively with cold water. In each case a solution of the metal hydroxide is produced together with hydrogen gas. The chemical equation for the reaction is.
2K 2H20 - 2KOH H2 endothermic reaction. 2K s 2H 2 O l 2KOH aq H 2 g heat. The metal belongs to the alkali group on the periodic table.
Potassium hydroxide is a basic oxide which all dissolve in water to form base solutionsPotassium hydroxide is actually the product of reacting potassium metal with water. In this case a solution of the metal hydroxide is produced together with hydrogen gas. ---- b Reaction of iron with steam - ----- c Hydrogen - gas is produced when dilute hydrochloric acid HCl is added to a reactive metal.