Great Example Of An Unbalanced Equation

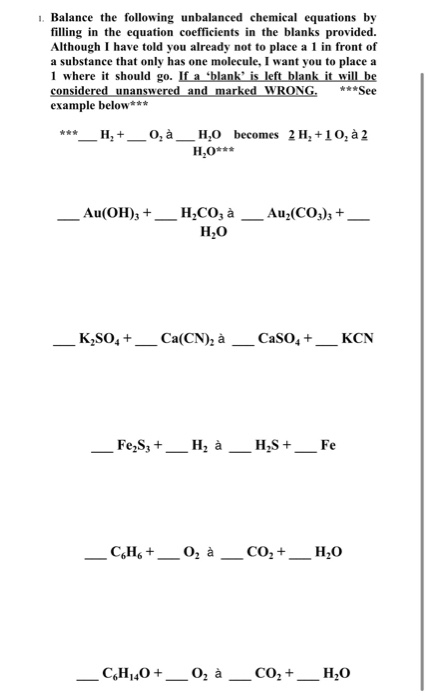
Balance the following equation.
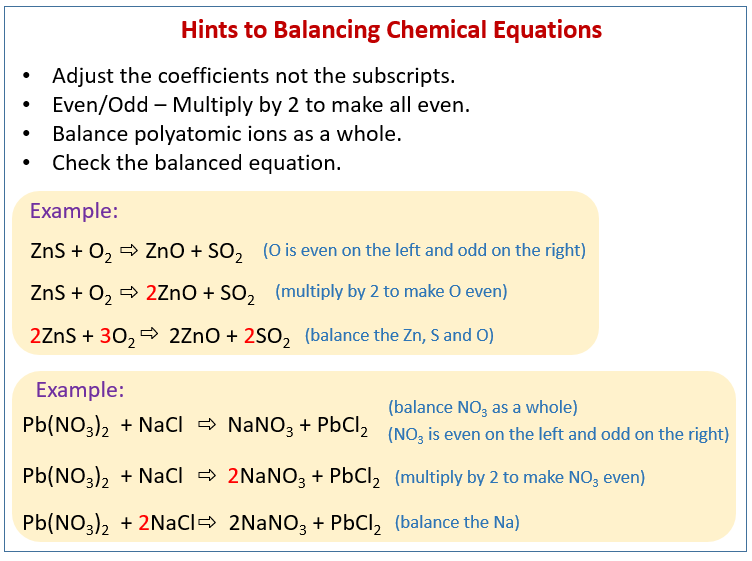
Example of an unbalanced equation. Unbalanced equation Na O 2 Na 2 O The reaction above between sodium and oxygen is not balanced. What is unbalanced equation with example. Note that if you have 1 of something it does not get a coefficient or subscript.
Fe 2 O 3 C Fe CO 2. Of the NH4OH on the left. It is more informative than the unbalanced chemical equation.
Then what is a balanced equation. The number of atoms is equal to the product of the coefficient and the subscript. The next most obvious unbalanced part is that there are now three NH4groups on the right but only one on the left hand side.
It states that mass can neither be created nor destroyed in a chemical reaction. Lets find out below and learn how to balance a chemical equation. For example consider the reaction of ethane C 2 H 6 with oxygen to yield H 2 O and CO 2 represented by the unbalanced equation.
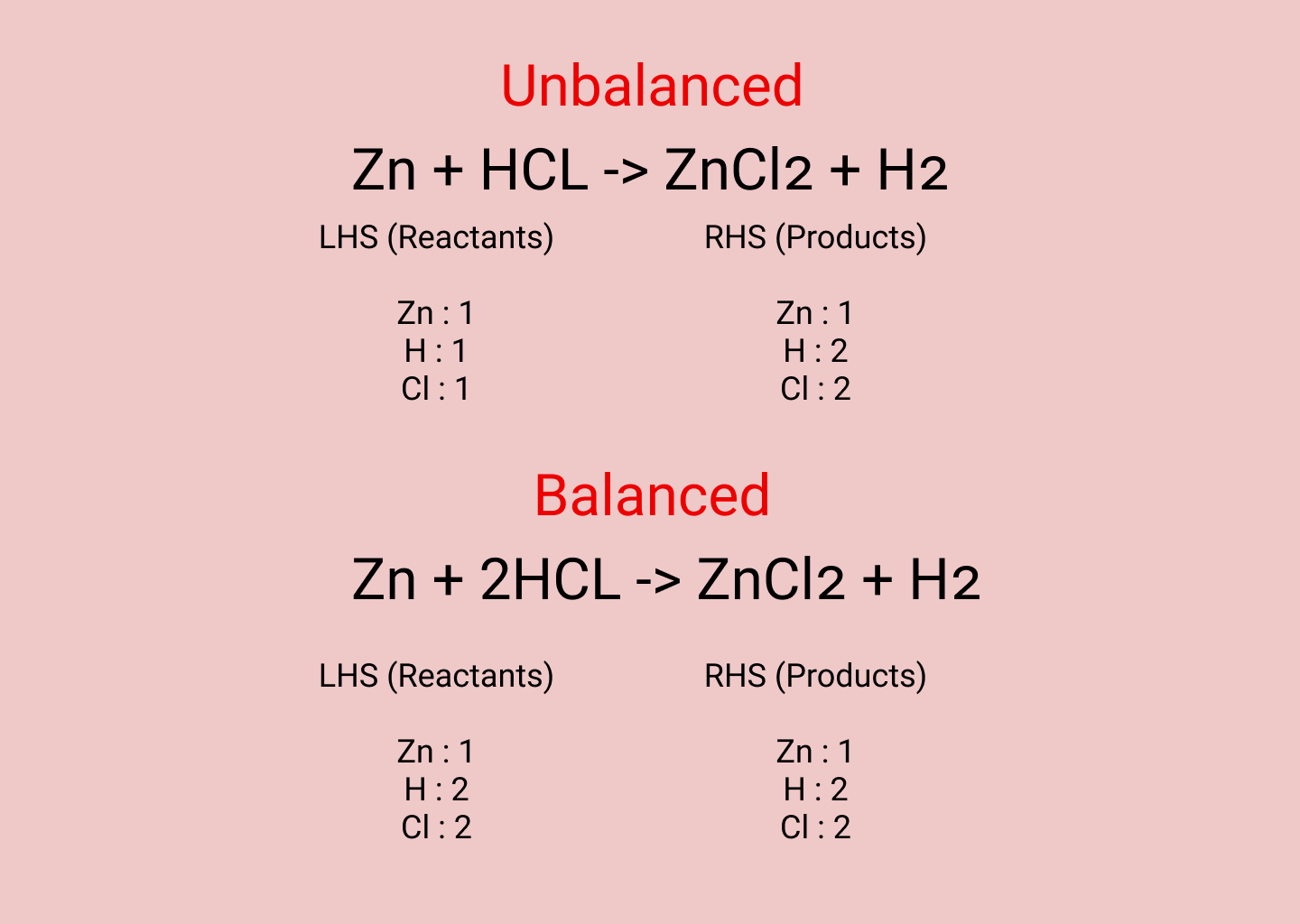
S ut5 at2 which is correct. We need to adjust the number of units of some of the substances until we get equal numbers of each type of atom on both sides of the arrows. In the reaction Mg O2 MgO the number of atoms of each element on either side of the arrow is not equal.
The word equations for a few of these reactions have been provided though most likely youll be asked to provide only the standard chemical equations. This means that there are UNEQUAL numbers at least one atom on each side of the arrow. FeCl3 3NH4OH --- FeOH3 3NH4Cl And if you count up the atoms on each side you will see that this is now.