Top Notch Equation For Combustion Of Gasoline

A complete combustion of acetylene gas C22 260 2 HO 2 This is balanced chemical equation b an absence of sufficient oxygen combustion does not occur completely.
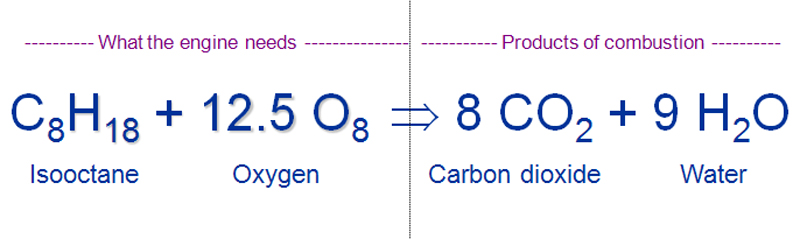
Equation for combustion of gasoline. The combustion may take place in a controlled manner such as in an internal combustion engine or industrial furnace or may result in an explosion eg a dust explosion gas or vapour explosion or in a thermobaric weapon. Gasoline is usually approximated as being made up of only octane whose chemical formula is C8H18. With unburned components in the exhaust gas such as C H 2 CO the combustion process is uncompleted and not stoichiometric.
Thats why engines need a source of oxygen-containing air and why engines emit carbon dioxide as a by-product of combustion. The thermochemical equation for the combustion of propane gas is as follows. Burning gasoline - When we burn gasoline we are combusting it or combining it with oxygen.
The numbers in front of each component of the reaction indicate the number of each molecule present. The balanced chemical reaction for octane burning is C8H1 8 125 O2 8 CO2 9 H2O. Thermodynamics is the study of energy and its transformations and describes the energetic properties of gases liquids solids or mixtures all this by understanding the relationship.
Barrans Jr Newton Ask-a-scientist. Gasoline ˈ ɡ æ s ə l iː n or petrol ˈ p ɛ t r ə l see the etymology for naming differences and the use of the term gas is a transparent petroleum-derived flammable liquid that is used primarily as a fuel in most spark-ignited internal combustion enginesIt consists mostly of organic compounds obtained by the fractional distillation of petroleum enhanced with a variety. In complete combustion of acetylene gas C2H2 C2 H 2 4 302 2 C0 HO 07 1 02 -Y 2 2C HO an a on complete combustion it gives coz while in by on incomplete.
As the reaction equation illustrates carbon. Ethanol is often used as an additive to gasoline. And the equation of the reaction of combustion of the diesel is.
The balanced equation for the combustion of methane shows that one molecule of methane reacts with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water. An ethanol addition up to 15 can be applied in conventional compression-ignition engines without the need for extensive engine modifications. The reaction of combustion of the gasoline is given by the following chemical equation.