Beautiful Decomposition Redox Reaction
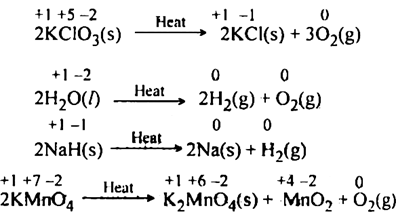
For a particular redox reaction NO is oxidized to NO3- and Cu2 is reduced to Cu.
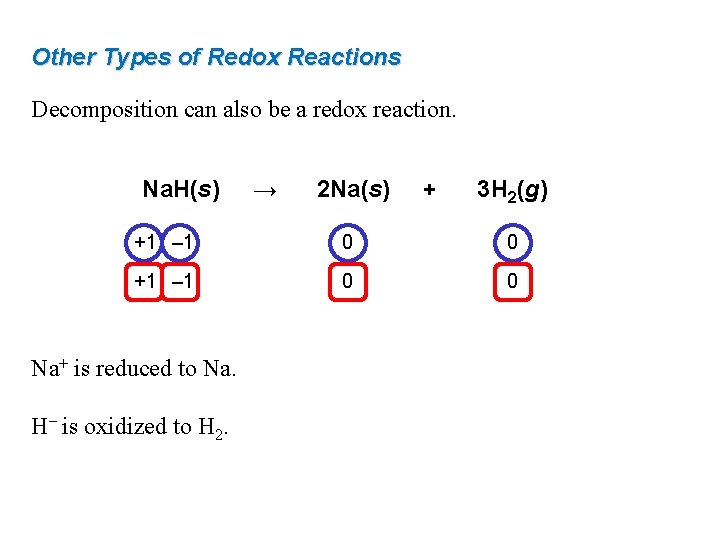
Decomposition redox reaction. If the decomposition reaction involves oxidation-reduction the reaction is often called an. Combination and decomposition Displacement reactions single and double Combustion Disproportionation. The chemical reaction in which both oxidation and reduction takes place is known as Redox reaction.
Combination reactions combine elements to form a chemical compound. Select all that apply. Combination decomposition displacement combustion and disproportion.
A _____ reactive metal can also displace the ion of. The oxidizing agent undergoes reduction and the reducing agent undergoes oxidation. 80 kJ mol21 allows the redox polymerization to be carried out under milder conditions than thermal polymerization.
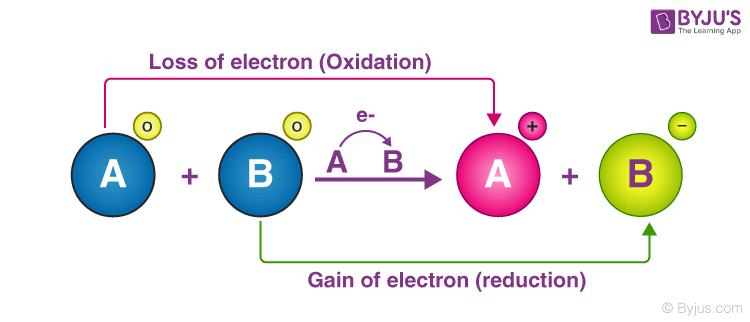
Keep this in mind as we look at the five main types of redox reactions. An oxidation-reduction redox reaction is a type of chemical reaction that involves a transfer of electrons between two species. For example in the following reaction Hydrogen is oxidised to become water and copper oxide is reduced to become copper.
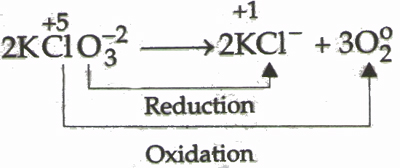
Decomposition of potassium chlorate is an example of redox reaction. One of these chemical reactions is known as the redox reaction which although it may not be mentioned much happens everywhere even in the human body. Balancing redox reactions is an important step that changes in neutral basic and acidic solutions.
Here hydrogen ive charge and oxygen ive charge are reduced and oxidized respectively during the reaction. Here Chlorine 5 oxidation state is reduced to Chlorine -1 while oxide -2 is oxidized to. In this review in addition to the classical examples of redox.