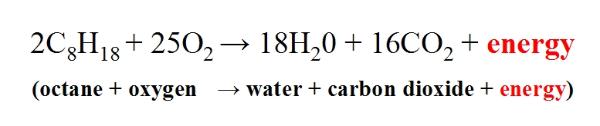Outrageous Combustion Reaction For Gasoline

Combustion is a reaction with oxygen.
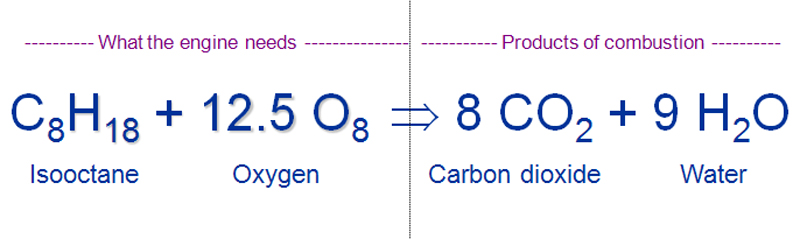
Combustion reaction for gasoline. This chemistry video tutorial explains how to balance combustion reactions. The chemical reaction equation for the combustion of octane C8H18 which is one of the primary components of gasoline is 2C8H18 25O2 16CO2 18H2O. A gasoline car typically uses a spark-ignited internal combustion engine rather than the compression-ignited systems used in diesel vehicles.
The combustion of a stoichiometric mixture of fuel and oxidizer eg. Combustion of fuel results from the equation of stoichiometry of oxygenfuel reaction. The general equation for a complete combustion reaction is.
It contains plenty of examples and practice problems on balancing combustion rea. In staged combustion eg burners-out-of-service and overfire air the degree of staging is a key operating parameter influencing NOx emission rates. In the combustion reaction the species reacting with the oxgyen is oxidized because oxygen is very electronegative.
Of the combustion of fossil fuels the combustion reaction is what we think of as a burning process. 1333 Nitrogen Oxides Emissions1-26-101517-27 -. For gasoline fuel generally used in spark-ignition and homogeneous charge compression ignition HCCI engines the Primary Reference Fuel mixture of n-heptane and iso-octane is often suggested as the surrogate mixture.
Gasoline fuel reacts with oxygen oxidant to produce carbon dioxide and water vapor. Fuel O 2 CO 2 H 2 O The fuel that burns in a combustion reaction usually consists of hydrocarbons which contain only carbon C and hydrogen H. Gasoline is a refined product of crude oil and is made up of many types of hydrocarbons.
When hydrogen and oxygen react during combustion water vapor is produced. Fossil fuels contain carbon C and hydrogen H. Development of Gasoline Combustion Reaction Model 2013-01-0887 Gasoline includes various kinds of chemical species.