Out Of This World Chemical Equation For Burning Gasoline

This reaction is known as combustion reaction.
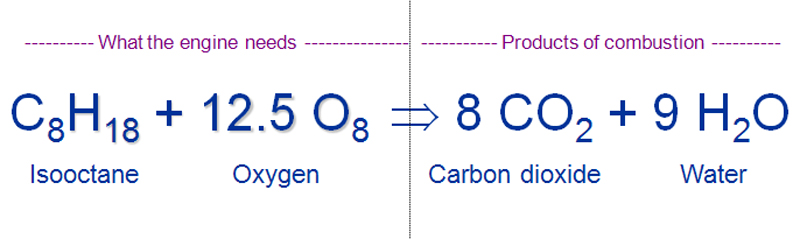
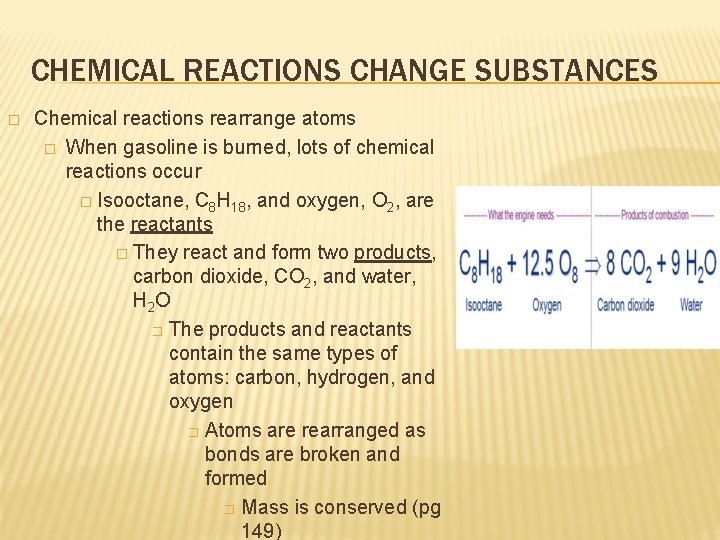
Chemical equation for burning gasoline. Write the balanced chemical equation for burning gasoline assume octane reacting with oxygen. The balanced chemical reaction for octane burning is C8H1 8. Then what is the equation for methane.
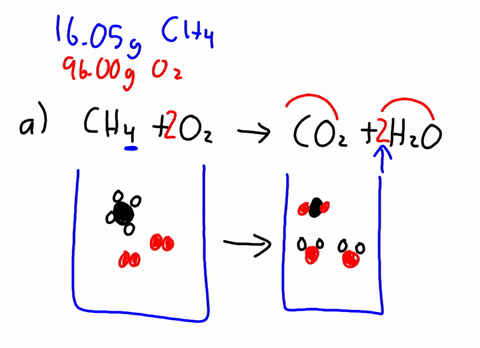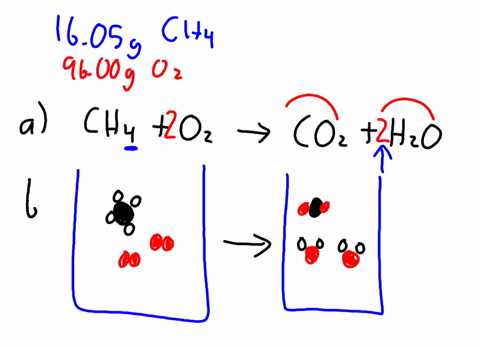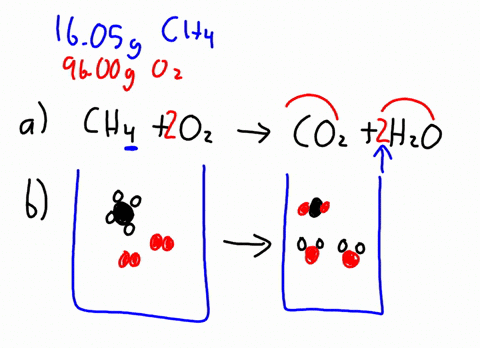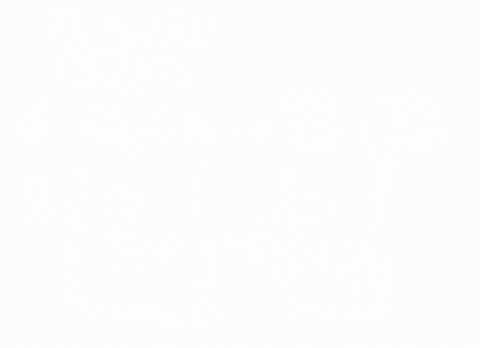
The combustion of methane is represented by the equation. The combustion of methane gas heats a pot on a stove. C8H18 O2 - CO2 H20 1 This is the same combustion reaction that occurs in our bodies to generate energy from food.
Determine the balanced chemical equation for this reaction. C 8 H 18 g O 2 g CO 2 g H 2 O g The octane rating of gasoline is a relationship of the burning efficiency of the given gasoline mixture to the burning efficiency of octane C 8 H 18. CH4 2O2 CO2 2H2O.
Barrans Jr Newton Ask-a-scientist. Gasoline is usually approximated as being made up of only octane whose chemical formula is C8H18. Hence the chemical equation for burning of methane is given above.
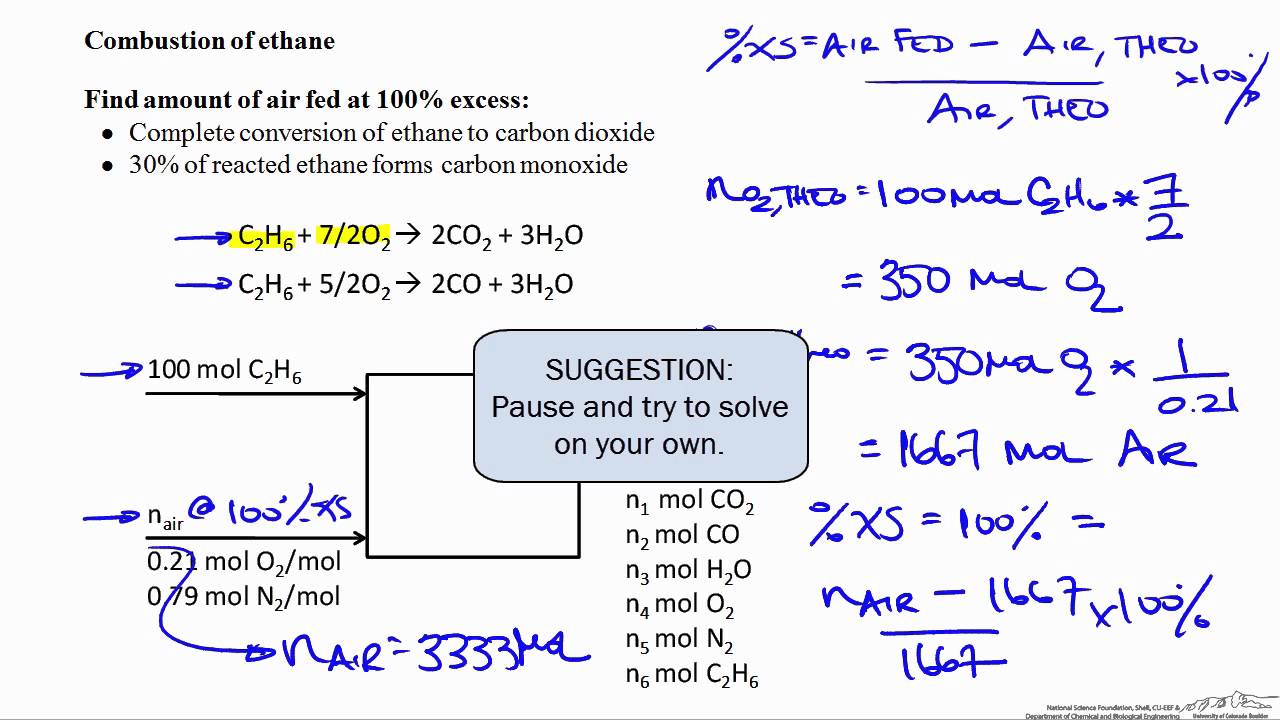
At httpecampusoregonstateeduchemistry you can earn college credit for online Chemistry and virtual labs. In complete combustion of acetylene gas C2H2 C2 H 2 4 302 2 C0 HO 07 1 02 -Y 2 2C HO an a on complete combustion it gives coz while in by on incomplete. As the soot is heated in the flame it produces a yellow flame.
1 mole of methane gas reacts with 2 moles of oxygen gas to produce 1 mole of carbon dioxide gas and 2 moles of water. Thats why engines need a source of oxygen-containing air and why engines emit carbon dioxide as a by-product of combustion. Methane is CH4 and when burnt in oxygen air it produces heat and CO2 and water.