Glory Balanced Equation For Ammonium Carbonate

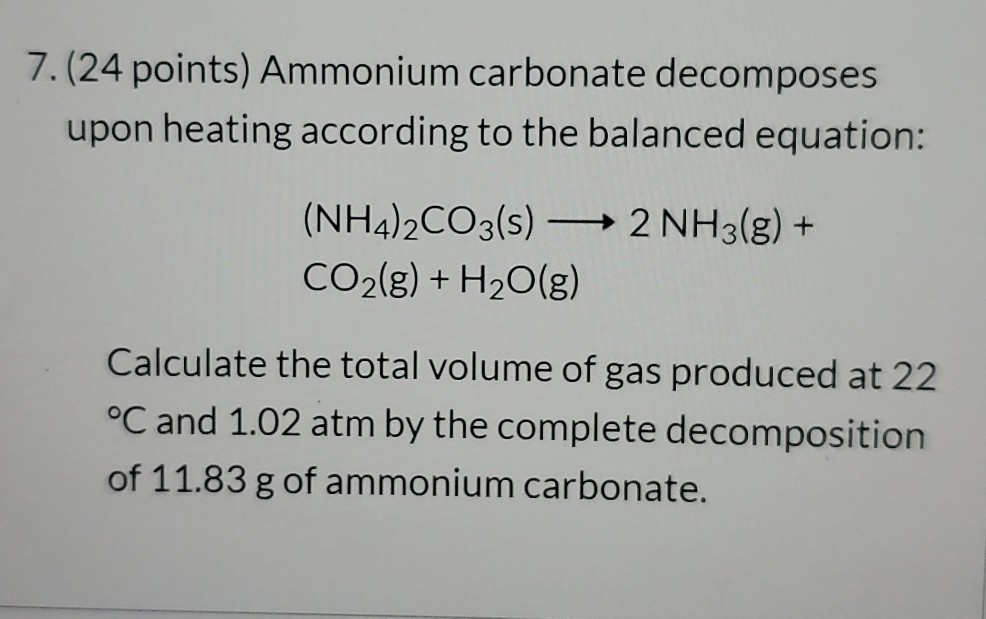
NH 4 2 CO 3 s 2 NH 3 g CO 2 g H 2 O g Calculate the total volume of gas produced at 200C and 103 atm by the complete decomposition of 118 g of ammonium carbonate.
Balanced equation for ammonium carbonate. Chemistry questions and answers. B Calculate the pressure of CO2 g atm gas present when 32 Lof ammonia gas is produced at 15 C. Suppose that ammonium carbonate is heated to 500 K in a sealed vessel.
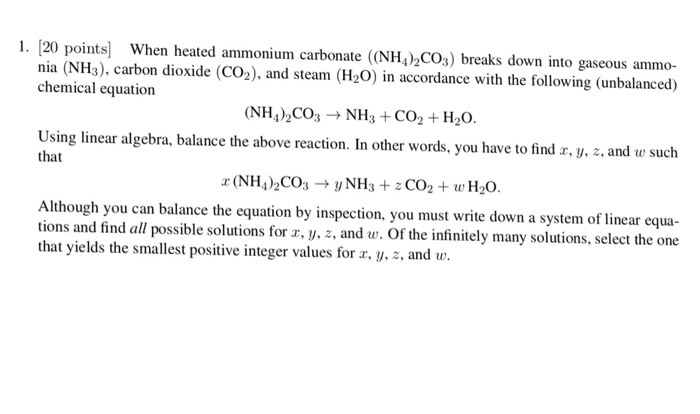
HCl aq NH42CO3 aq NH4Cl aq H2CO3 aq Since all products are still soluble assuming this reaction takes place in. Ammonium carbonate decomposes upon heating according to the following balanced equation. CaCO 3 CO 2 CaO 17.
Get the detailed answer. Ammonium carbonate decomposes upon heating according to the balanced equation. The primary hazard is the threat to the environment.
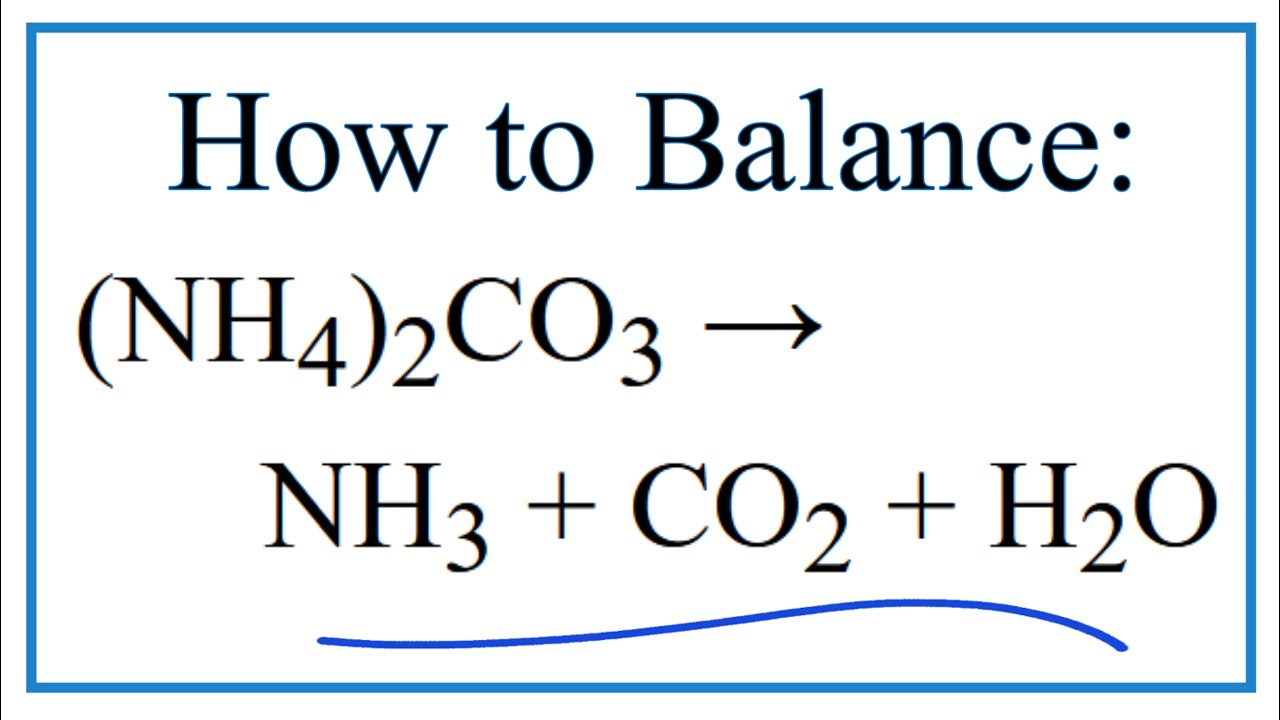
Ammonium carbonate solid decomposes when heated to produce three gaseous products - ammonia water and carbon dioxide. When solid ammonium carbonate is heated it decomposes into ammonia gas NH 3 carbon dioxide gas and water vapor. Better learning for better results.
Question 22 of 40 Complete the balanced molecular chemical equation for the decomposition reaction that takes place as solid ammonium carbonate when heated. What is the balanced equation for ammonium carbonate is heated it decomposes into ammonia gas carbon dioxide gas and water vapo Free unlimited access for 30 days limited time only. The chemical formulas for barium nitrate and ammonium carbonate are Ba NO32 and NH42CO3.
Calcium carbonate carbon dioxide calcium oxide Unbalanced equation. What is the balanced equation and type of reaction when you mix a solution of ammonium carbonate and leadIV acetate. NH42CO3 Then write an unbalanced equation.