Ace Balanced Combustion Reaction For Ethane

In order to balance C2H6 O2 CO2 H2O youll need to watch out for two things.
Balanced combustion reaction for ethane. Ethane C2H6 reacts with oxygen O2 to make carbon dioxide CO2 and water H2O. Propane undergoes combustion reactions in a similar fashion to other alkanes. Consider the combustion of ethane.
Write the word equation for the reaction of barium nitride Ba3N2 with potassium. To balance a combustion reaction we first balance the number of carbons. Ethane is an alkane with the chemical formula C2H6.
During the combustion of 500 g of ethane C2H6 355 kcal is released a Write a balanced. What is the balanced equation for the combustion of ethane. During the combustion of 500 g of ethane C2H6 355 kcal is released a Write a balanced equation for the combustion of ethane b What is the sign of H for this reaction.
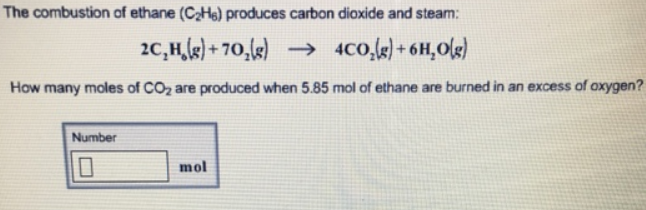
Arrange the reactions given below in proper sequence for the conversion of ethane to methane. Explain why alkanes and alkenes are oxidised during complete combustion. 2C2H6 g7O2 g--4CO2 g6H2O g If the ethane is burning at the rate of 07 molL s at what rates are and being produced.
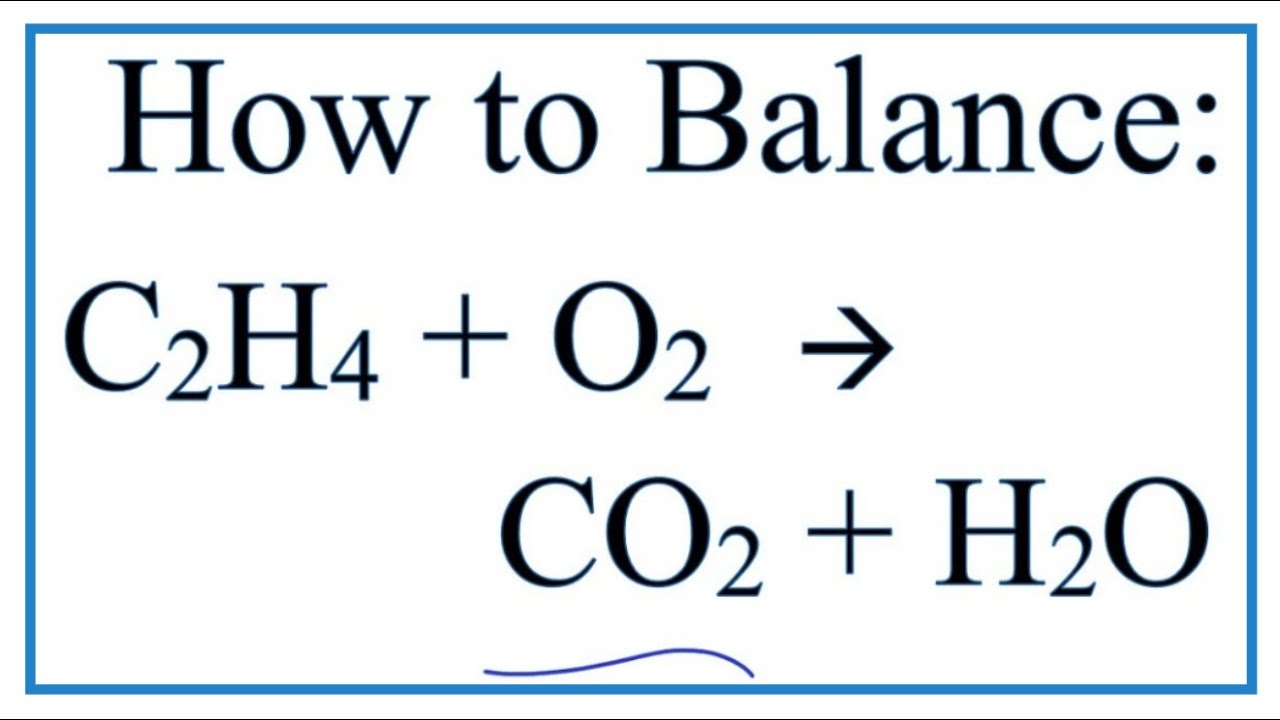
2 C2H2 5 O2 4 CO2 2 H2O. Is C2H4 3O2 2CO2 2H2O balanced equation. There are 2 carbon atoms from ethene and only one carbon atom from carbon dioxide CO2 C O 2.
Fuel O2 CO2 H2O You would then balance the chemical equation. The balanced chemical equation for the complete combustion of ethane is. 2 C 2 H 6 7 O 2 4 CO 2 6 H 2 O Heat Energy Enthalpy The hydrocarbon combustion reaction releases heat energy and is an example of an exothermic reaction.